Australia Makes $1 Billion Bet On Coming H5N1 Pandemic, Creates Bird Flu Task Force
Follows U.S. allocation of $1 billion for future bird flu pandemic in March omnibus bill.
The Australian government has unveiled a massive $1 billion biosecurity investment to prepare for potential outbreaks of the H5N1 bird flu, a strain it says poses “significant risks” to the nation’s industry, trade, and wildlife.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
This is despite Australia being “the only continent to have not detected H5N1,” according to a September report from News.com.au, a prominent Australian news website owned by News Corp Australia.
“Australia remains the only continent free of this H5N1 HPAI strain, and our government will continue to focus on ensuring Australia is prepared for any future detection,” Agriculture Minister Julie Collins said. “We have recently invested an extra $7m to focus on the threats posed by this strain of avian influenza, and more than $1bn in new biosecurity funding overall has been invested by our government.”
“The situation remains dynamic and we are committed to provide an agile intergovernmental, industry and community response to protect Australian industry, trade and wildlife,” she added.

In addition to the massive funding, Australia has created a task force across government departments, and “stress-tested its preparedness” in August and September with a series of H5N1 outbreak simulation exercises, Reuters reported in early October.
U.S. Prepares for Next Pandemic: Over $1 Billion Allocated for Bird Flu Amid Parallels to COVID-19 Response
Australia’s investment came only months after the United States enacted legislation allocating over $1 billion for a future bird flu pandemic.
As previously reported by this website, Joe Biden signed into law the massive $1.2 trillion “Omnibus” spending package in March.
The 1,012-page bill passed the Senate in a 74 to 24 vote after it advanced from the House 286 to 134.
The legislation specifically allocated $708,272,000 for “emerging and zoonotic infectious diseases.”
‘Emerging’ refers to newly identified or evolving infectious diseases, while ‘zoonotic’ infectious diseases are infections that can be transmitted between animals and humans, like COVID-19.
“For carrying out titles II, III, and XVII, and section 2821 of the PHS Act, and titles II and IV of the Immigration and Nationality Act, with respect to emerging and zoonotic infectious diseases, $708,272,000” are allocated, page 489 of the bill reads.
While this section of the bill does not identify a specific zoonotic disease by name, a later section earmarks another $315,000,000 for a coming “influenza (flu) pandemic,” including “the production of pandemic influenza vaccines,” comprising a total of $1,023,272,000 for a future bird flu outbreak.
More Echoes of COVID: USDA’s Risky Bird Flu Experiments
In events eerily reminiscent of the lead-up to the COVID pandemic, the Omnibus Bill was passed following dangerous gain-of-function experiments on H5N1 bird flu viruses conducted by the U.S. Department of Agriculture (USDA) in collaboration with China.

However, while conducting these risky bird flu experiments, the USDA was also simultaneously developing a vaccine for the H5N1 influenza strain, raising questions about government knowledge and intentions as well as bioweapons concerns.
Both the U.S. Department of Energy and the Federal Bureau of Investigation (FBI) have concluded that the COVID pandemic most likely arose from a laboratory leak at China’s Wuhan Institute of Virology (WIV), where the National Institutes of Health (NIH) admitted it did fund risky gain-of-function research.
Pandora’s Box Opened: New Policy Lets Agencies Skip Safety Protocols for Deadly Virus Experiments
Alarmingly, new rules issued by the U.S. Office of Science and Technology Policy (OSTP) permit the Secretary of “any federal department” to waive oversight requirements for high-risk research involving pathogens with “enhanced” pandemic potential for up to 180 days, with the option for renewals—effectively allowing risky gain-of-function research to bypass standard safety checks.
That new policy, published without any media attention on the White House website in May, authorizes any federal agency to waive standard safety protocols for experiments that even involve resurrecting eradicated viruses like 1918 influenza (Spanish Flu) and smallpox.
No Need for Bird Flu Vaccine EUA—Xofluza and Ivermectin Already Work Against the Virus
The U.S. Food and Drug Administration (FDA) has confirmed the agency will again skip the rigorous drug approval process for influenza bird flu vaccines, as it did for COVID jabs, utilizing the controversial Emergency Use Authorization (EUA) tactic.
EUAs allow drugs with no long-term safety data to be distributed to the public without receiving full FDA approval.
However, EUAs may “only be granted when no adequate, approved, available alternatives exist,” according to Yale Medicine.
This website has reported that antivirals like Xofluza and broad-spectrum anti-parasitics like Ivermectin are already fully FDA-approved, safe, and effective medicines against bird flu, thus removing the need for a bird flu vaccine EUA.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
Biden Policies for Gain-of-Function Experiments on Deadly Extinct Viruses Including Spanish Flu and Smallpox Effective 2025
The Biden administration has quietly introduced new rules, effective in 2025, that dramatically reduce government oversight on high-risk pathogen research, the same research the COVID-19 pandemic is said to have arisen from.
8 Studies Support Ivermectin's Effectiveness Against Influenza Amid Bird Flu Pandemic Worries
As the H5N1 influenza A virus (IAV) infects birds and mammals, including humans, across the world, health experts are warning a bird flu pandemic could be “100 times worse” than COVID-19.
7 Studies Confirm Antiviral 'Xofluza' Is 'Drug of Choice' for Bird Flu, as USDA Tests Ground Beef for Virus
The U.S. Department of Agriculture (USDA) will begin testing ground beef for H5N1 bird flu (influenza) particles, as the virus has reportedly been found in nearly three dozen dairy herds across nine states.
NIH Admits Funding Gain-of-Function Research in Wuhan, China (Video)
Dr. Lawrence A. Tabak, Principal Deputy Director of the National Institutes of Health (NIH), on Thursday admitted before the U.S. House of Representatives Select Subcommittee on the Coronavirus Pandemic that his agency did fund risky gain-of-function research in Wuhan, China.
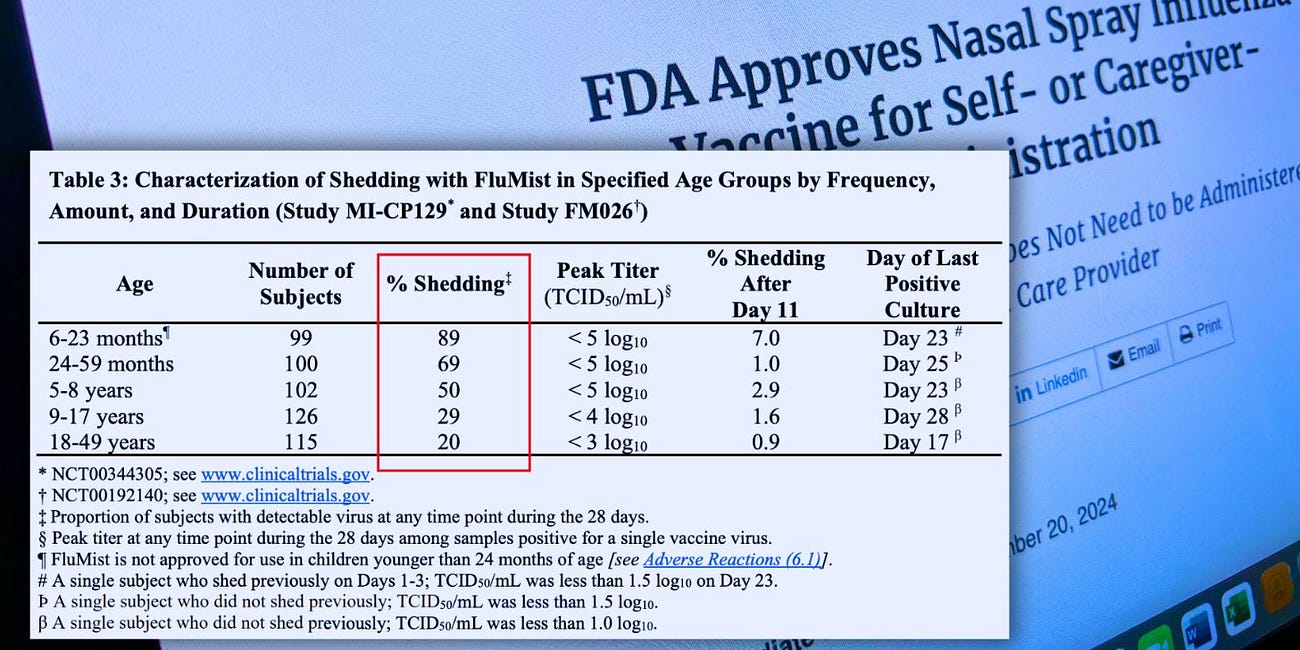
FDA Approves AstraZeneca No-Needle At-Home 'Live' Virus Flu Vaccine with 90% Shed Rate
In September, the U.S. Food and Drug Administration (FDA) approved AstraZeneca’s FluMist for self or caregiver administration for the 2024-2025 influenza season.
'Wuhan West' Colorado State University Bat Lab Receives Millions in New NIH Funding
In a timely revelation during Halloween’s Bat Week (October 24–31), new federal funding has been allocated to Colorado State University’s (CSU) bat research facility in Fort Collins, Colorado, stoking opposition from public health advocates and government officials alike.
Operation 'Large Area Coverage' (LAC): U.S. Gov't Covertly Sprays Airborne Toxic Chemical Agent on Unsuspecting Americans in Secret Bioweapons Experiment
In the 1950s and 1960s, Stanford University and the U.S. Army Chemical Corps conducted aerial dispersion tests using military transport aircraft that exposed unsuspecting Americans to fluorescent zinc cadmium sulfide (ZnCdS) particles, as part of …
U.S. 'Relaxes' Biolab Regulations for Handling Deadly Pathogens Despite Ex-CDC Chief's Bird Flu Pandemic Warning
The United States has “temporarily relaxed strict guidelines” for handling, storing, and transporting dangerous H5N1 bird flu samples, following a request from the Association of Public Health Laboratories (APHL), a U.S. government-funded nonprofit membership organization.
U.S. Gov't Manufactures Bird Flu PCR Test In-House Without Third-Party Oversight
The United States government has come under scrutiny for manufacturing the bird flu PCR test in-house without third-party oversight, raising concerns about the accuracy and reliability of the tests as well as their purpose.
Treaty 'Loophole' Allows World Governments to Develop Deadly Bioweapons Like COVID and Corresponding Vaccines: Yale Professor (Video)
The ‘Convention on the Prohibition of the Development, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and On Their Destruction,’ known as the “Biological Weapons Convention” (BWC), was opened for signature in April 1972 and took effect in March 1975.
American Bioweapons: Then & Now
The United States had an extensive offensive biological weapons program stretching back at least to World War II and the Cold War era.
Vaccines Are DOD 'Bioweapons': Global Conspiracy Using Public Health to 'Kill Lots of People' Constitutes 'War Crimes' (Video)
In a series of troubling assertions, Katherine Watt of Bailiwick News, a writer and paralegal, alleges that a global campaign is being conducted under the guise of public health measures, aimed at population control and mass harm.
















Canada is trying to do the same. Take Action to STOP Canada's Bill C-293 An Act respecting pandemic prevention and preparedness
.
https://StopC-293.ca
.
Re: your article’s last section discussing the topic of the EUA: Katherine Watt has pointed out that ALL pharmaceutical products — not only vaccines but also prescribed medications such as pills, etc. — have been covered under a blanket EUA since October 2021 (that date might even be October 2020; I’ll have to check).