FDA Approves AstraZeneca No-Needle At-Home 'Live' Virus Flu Vaccine with 90% Shed Rate
FluMist manufacturer insert confirms vaccinated can infect unvaccinated.
In September, the U.S. Food and Drug Administration (FDA) approved AstraZeneca’s FluMist for self or caregiver administration for the 2024-2025 influenza season.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
AstraZeneca subsidiary MedImmune, LLC, the manufacturer of FluMist, anticipates that FluMist will be available for the 2026 influenza season as well.
FluMist, which is sprayed into the nose, is now approved for the alleged prevention of influenza disease caused by influenza virus subtypes A and B in individuals 2 through 49 years of age.

Each refrigerated FluMist sprayer contains a single 0.2 mL dose with “live” attenuated influenza virus (10^6.5–7.5 FFU) from three strains: A/Norway (H1N1), A/Thailand (H3N2), and B/Austria (B/Victoria lineage).

Alarmingly, the FDA package insert indicates that the vaccinated can shed (or transmit) the vaccine virus onto the unvaccinated, potentially infecting them.
“Vaccine viruses capable of infection and replication can be cultured from nasal secretions obtained from vaccine recipients,” the document reads.
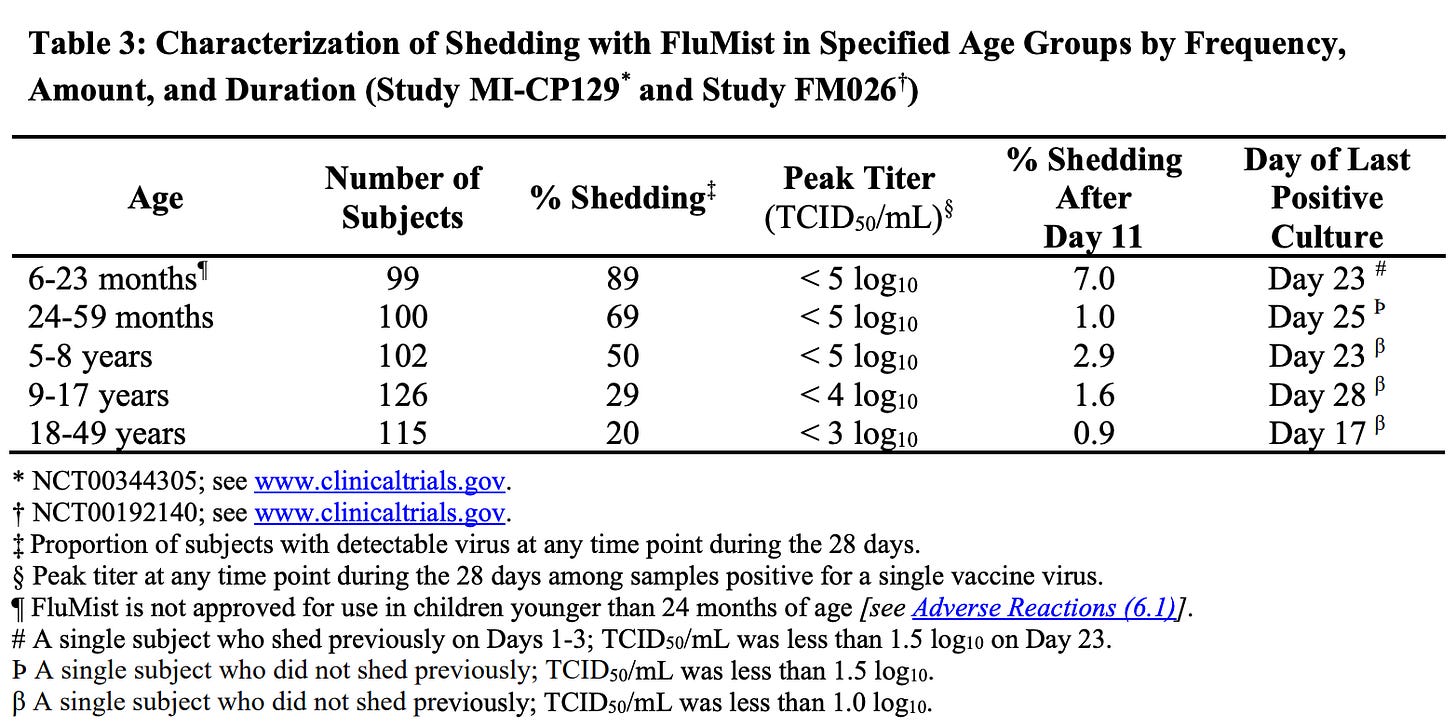
Vaccine virus shedding within 28 days of FluMist vaccination was studied in two multi-center trials: Study MI-CP129 (200 healthy participants aged 6 to 59 months) and Study FM026 (344 healthy participants aged 5 to 49 years).
In both studies, nasal samples were collected daily for the first 7 days, then every other day through Day 28.
In Study MI-CP129, participants with positive shedding on Day 25 or Day 28 had additional samples taken weekly until two consecutive samples tested negative.
Here’s a breakdown of the percentage of individuals in different age groups who tested positive for the FluMist vaccine virus at any point within 28 days of vaccination:
6-23 months: 89% tested positive for the virus
24-59 months: 69% tested positive
5-8 years: 50% tested positive
9-17 years: 29% tested positive
18-49 years: 20% tested positive

The FDA insert cites a study in a daycare setting that tested whether FluMist vaccine viruses can spread from vaccinated to unvaccinated children under age 3.
It confirmed that the vaccine can infect others, with documented transmission of a Type B virus from a vaccinated child to an unvaccinated child in the same playgroup.

You can read the full FDA vaccine insert for FluMist below:
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
Ebola Vaccine That 'Sheds' in 31% of Vaccinated Given to Colorado Healthcare Workers Just Down the Road from New Bat Lab
Editor’s note: This article’s headline has been updated to clarify that the FDA insert for Merck’s ERVEBO Ebola vaccine states that the vaccine “sheds” in more than 31% of those vaccinated with the drug. The prior headline said that the vaccine “‘Sheds’ Onto/Infects Others 31% of the Time.” It is more precise to simply say that the vaccine “sheds” in 31…
COVID Vaccinated Could Shed Lipid Nanoparticles, Spike Protein Through Blood Transfusion, Breastmilk, Organ Transplantation, Exhalation, Skin-to-Skin Contact: New Preprint Study
A new study made available online today in preprint analyzes exposure to COVID-19 vaccine components such as lipid nanoparticles or spike protein.
Death Reported in Unvaccinated Individuals Infected by Those Vaccinated for Monkeypox: FDA Package Insert
The monkeypox vaccine ACAM2000 contains a live virus that the vaccinated can spread to and even kill those who have not received the jab, according to the U.S. Food and Drug Administration (FDA).
Bird Flu: CPT Code Update Readies U.S. Health Systems for New mRNA Jab Rollout Skipping FDA Approval Process
In anticipation of a coming avian influenza (bird flu) pandemic, the American Medical Association (AMA) on Friday announced an editorial update to the ‘Current Procedural Terminology’ (CPT) code set in order to account for the development and administration of bird flu “vaccines.”
U.S. Gov't Manufactures Bird Flu PCR Test In-House Without Third-Party Oversight
The United States government has come under scrutiny for manufacturing the bird flu PCR test in-house without third-party oversight, raising concerns about the accuracy and reliability of the tests as well as their purpose.
FDA to Skip Drug Approval Process for Bird Flu Vaccine: Dr. Peter Marks Confirms Agency Will Leverage Controversial 'Emergency Use Authorization' (EUA) Tactic as It Did with Deadly COVID Jab
Dr. Peter Marks, Director of the Center for Biologics Evaluation and Research (CBER) at the U.S. Food and Drug Administration (FDA), confirmed last week that the FDA will skip the rigorous drug approval process for influenza bird flu (H5N1) vaccines, as it did for COVID-19 jabs.
PCR Test Now Being Used to Detect Bird Flu Is 97% Unreliable: Oxford Academic Journal 'Clinical Infectious Diseases'
A September 2020 publication in Clinical Infectious Diseases confirms that the test method known as ‘reverse-transcription polymerase chain reaction’ (RT-PCR), currently being used to detect the presence of the bird flu virus, is accurate less than 3% of the time.











Just what we need...more sheep shedding not only spike protein and Lord knows what else! 🥴🙄
Holy spike proteins batman!!!