'Gene Therapy' Cancer Drug That Costs $727,000 Per Treatment and Only Works 'Completely' 4% of the Time Granted 'Accelerated Approval' by FDA
Adaptimmune's "genetically modified" tumor drug TECELRA contains "short spans of genetic material that are identical to HIV."
The U.S. Food and Drug Administration (FDA) on Friday approved Adaptimmune’s first-of-its-kind gene therapy to treat a rare type of solid tumor cancer in the soft tissues typically affecting young men.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
The genetically modified drug, TECELRA® (afamitresgene autoleucel), is for the treatment of adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy.
“TECELRA is the first engineered cell therapy for a solid tumor cancer approved in the U.S., and the first new therapy option in more than a decade for synovial sarcoma, a rare, soft tissue cancer that most commonly impacts young adults,” a press release from Adaptimmune reads.
Synovial sarcoma most commonly develops in the extremities and impacts about 1,000 Americans each year, often affecting adult males in their 30s or younger.
The gene therapy is administered as a single intravenous dose.
The therapy is composed of a patient’s own T cells that have been genetically modified to express a T cell receptor (TCR) targeting the MAGE-A4 antigen.
The process is said to involve collecting T cells from the patient, modifying these cells to recognize and attack cancer cells expressing the MAGE-A4 antigen, and then reintroducing the modified T cells back into the patient.
The company says the treatment will launch at a list price of $727,000.
The FDA granted TECELRA “accelerated approval,” which is given to drugs for “serious conditions that fill an unmet medical need to be approved based on a surrogate endpoint,” according to the agency’s website.
Accelerated approval enables the FDA “to approve these drugs faster,” bypassing the regulator’s more rigorous full approval process.
Despite being the first gene therapy to be approved in the United States that uses a patient’s own immune response generating T-cells to fight cancer, the accelerated approval was based on a single seven-month study published in The Lancet.
And that study only included 44 patients.
“The approval of TECELRA was based on results of the SPEARHEAD-1 (Cohort 1) trial, which included 44 patients,” the press release reads.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
Moreover, the study confirmed only about a third of patients experienced any positive response to the treatment.
“Overall response rate was 37% (19 of 52; 95% CI 24–51) overall, 39% (17 of 44; 24–55) for patients with synovial sarcoma, and 25% (two of eight; 3–65) for patients with myxoid round cell liposarcoma,” the Lancet study says.
Significantly, only 4.5% experienced a “complete response.”
The study also revealed a potentially life-threatening systemic inflammatory response called ‘cytokine release syndrome’ occurs in most (71%) of patients.
The press release reads: “BOXED WARNING: Cytokine release syndrome (CRS), which may be severe or life-threatening, occurred in patients receiving TECELRA. At the first sign of CRS, immediately evaluate patient for hospitalization and institute treatment with supportive care. Ensure that healthcare providers administering TECELRA have immediate access to medications and resuscitative equipment to manage CRS.”
Following treatment, two percent of patients experienced ‘Immune Effector Cell–associated Neurotoxicity Syndrome’ (ICANS), a serious neurological side effect.
Infections occurred in a third (32%) of patients.
Some patients treated with TECELRA even developed “secondary malignancies or recurrence of their cancer.”
Under a section titled “Potential for HIV Nucleic Acid Test False-Positive Results,” Adaptimmune explains that TECELRA contains “short spans of genetic material that are identical to HIV” and that therefore “some commercial HIV nucleic acid tests may yield false-positive results in patients who have received TECELRA.”
The company advises patients should “not donate blood, organs, tissues, or cells for transplantation” for “at least 4 weeks” after receiving TECELRA.
Finally, TECELRA contains Dimethyl Sulfoxide (DMSO), a substance known to induce genetic mutations, which are permanent changes in an organism’s DNA.
DMSO may be toxic to blood, kidneys, liver, mucous membranes, skin, eyes, according to one safety data sheet.
“Repeated or prolonged exposure to the substance can produce target organs damage,” the document warns.
Adaptimmune is owned by the infamous asset manager BlackRock, an official partner of the World Economic Forum (WEF).
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
COVID Spike Protein—Present in Pfizer, Moderna mRNA Jabs—Helps Cancer Cells Survive Chemotherapy: Brown University Study Preprint in 'BioRxiv'
Brown University researchers posted a preprint study last month in BioRxiv, an open-access preprint repository for the biological sciences, confirming that the SARS-CoV-2 spike protein interferes with the effectiveness of chemotherapy treatment for cancer.
mRNA COVID Jab Ingredient N1-Methyl-Pseudouridine (m1Ψ) 'Stimulated Cancer Growth and Metastasis': 'International Journal of Biological Macromolecules'
A new study published earlier this month in the International Journal of Biological Macromolecules confirms that an ingredient in mRNA COVID-19 injections called N1-methyl-pseudouridine (m1Ψ) increases cancer growth and spread.
Excess Cancer Deaths Spike from -1,379 in 2020 to +7,162 in 2022 'After Mass Vaccination' with mRNA COVID Jabs in Japan: Journal 'Cureus'
A brand new peer-reviewed study published in Cureus on Monday found significant increases in age-adjusted mortality rates of all cancer types in 2022 after the majority of the Japanese population received doses of the COVID-19 mRNA vaccine.
Man Suffers Skin Cancer, Heart Disease Following COVID-19 Vaccinations: 'Frontiers in Oncology' Case Report
A new case report published this month in the peer-reviewed journal Frontiers in Oncology confirms a Japanese man suffered from metastatic malignant melanoma and heart failure following COVID-19 vaccination.
Repeat COVID-19 Vaccinations Linked to 'Multi-Hit' Cancer Growth Model: Peer-Reviewed Journal 'Cureus'
A study published last month in the peer-reviewed journal Cureus has linked COVID-19 vaccines to the “multi-hit hypothesis” of cancer growth in the body.
Cancer Reappears in Patient Following COVID-19 Vaccination: Peer-Reviewed Journal 'Infection' Case Study
A new publication in the peer-reviewed medical journal Infection reported the reappearance of Kaposi’s sarcoma (KS), a type of cancer that develops from the cells that line lymph or blood vessels, in a 48-year-old male patient who recently received multiple COVID-19 vaccinations.
COVID-19 Vaccines May Pose 'Unique and Heightened Risk' of Contaminated DNA Fragment Integration into Human Body: Florida Surgeon General
In a strongly worded letter dated December 6, 2023, Florida Surgeon General Joseph A. Ladapo raised significant concerns to the U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) regarding the safety and efficacy of COVID-19 vaccines.
COVID Jab Spike Protein Remains in Body 'Up to 245 Days'—Not a 'Few Weeks' as Health Authorities Claimed: Journal 'MedRxiv'
A March publication in the peer-reviewed medical journal MedRxiv confirms that the spike protein produced by cells after mRNA COVID-19 injection remains in the body for “up to 245 days,” contradicting claims from mainstream health authorities.
Pfizer COVID Jab's Dangerous DNA Impurities 'Exceed the Permitted Limit Value' by 'More Than 500 Times': Peer-Reviewed Journal 'Methods and Protocols'
A new study published this month in MDPI’s peer-reviewed Methods and Protocols confirms Pfizer Inc.’s COVID-19 injection contains in some cases “more than 500 times” the permitted limit of potentially cancer-causing DNA contamination.
More Vaccinations Means 'Exponentially' More Disease in Infants: 'International Journal of Vaccine Theory, Practice, and Research'
A new study published Wednesday in the peer-reviewed International Journal of Vaccine Theory, Practice, and Research confirms infants who receive more vaccinations have “exponentially” more diseases.
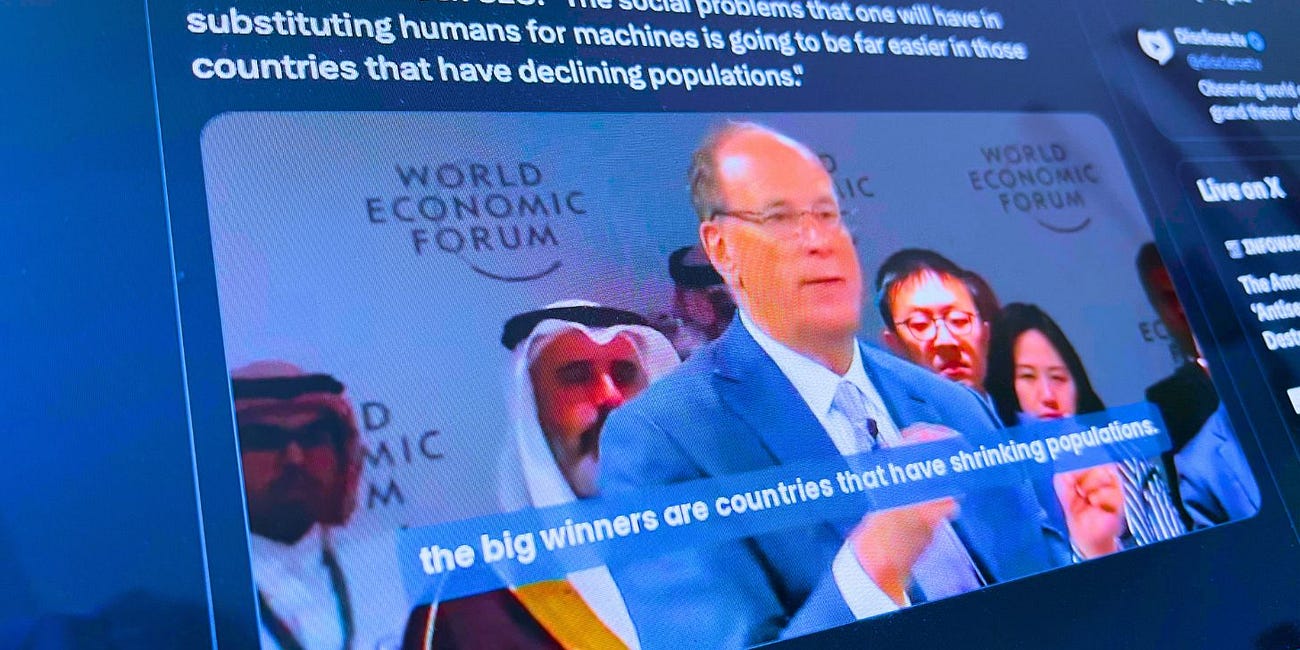
Depopulation Agenda Endorsed by WEF-Allied BlackRock CEO Larry Fink: 'Shrinking Populations' Better for 'Robotics and AI' Takeover (Video)
BlackRock Inc. CEO Larry Fink praised depopulation, an idea associated with reducing the number of people in a country or region, in remarks made during the World Economic Forum’s (WEF) recently held ‘Special Meeting on Global Collaboration, Growth and Energy Development’ in Saudi Arabia.
The 'WEF Axis' of Evil: How The World Economic Forum Controls Everything You Do
The World Economic Forum (WEF) is behind the global climate change narrative, promoting China as the next world superpower, and worldwide Diversity, Equity, and Inclusion (DEI) efforts.
List of Every Company Officially Partnered With the World Economic Forum (WEF)
The 54th Annual Meeting of The World Economic Forum (WEF) is currently underway at Davos-Klosters in the Swiss canton of Graubünden.
World Economic Forum Is 'Toxic Workplace' Facing 'Numerous Accusations of Sexual Harassment and Discrimination Against Women and Black People': WSJ
Despite its mission to “improve the state of the world,” the World Economic Forum (WEF), best known for its annual Davos summit, is facing serious allegations of sexual harassment, discrimination, and a toxic workplace culture under the leadership of its founder, Klaus Schwab.
















I wonder who the investors are? Politicians?
If like me you are sick of being abused by your so called leaders you now have the option to leave the system and it is completely lawful.
In fact by paying taxes you are now funding war crimes, crimes against humanity and genocide.
Many more details here; https://truthaddict.substack.com/p/how-to-exit-the-matrix