FDA Greenlights New Bill Gates-Funded ARCT-2304 Self-Replicating samRNA 'Pandemic' H5N1 Bird Flu Jab
Drug maker Arcturus "is actively engaged with the U.S. government to prepare for the next pandemic."
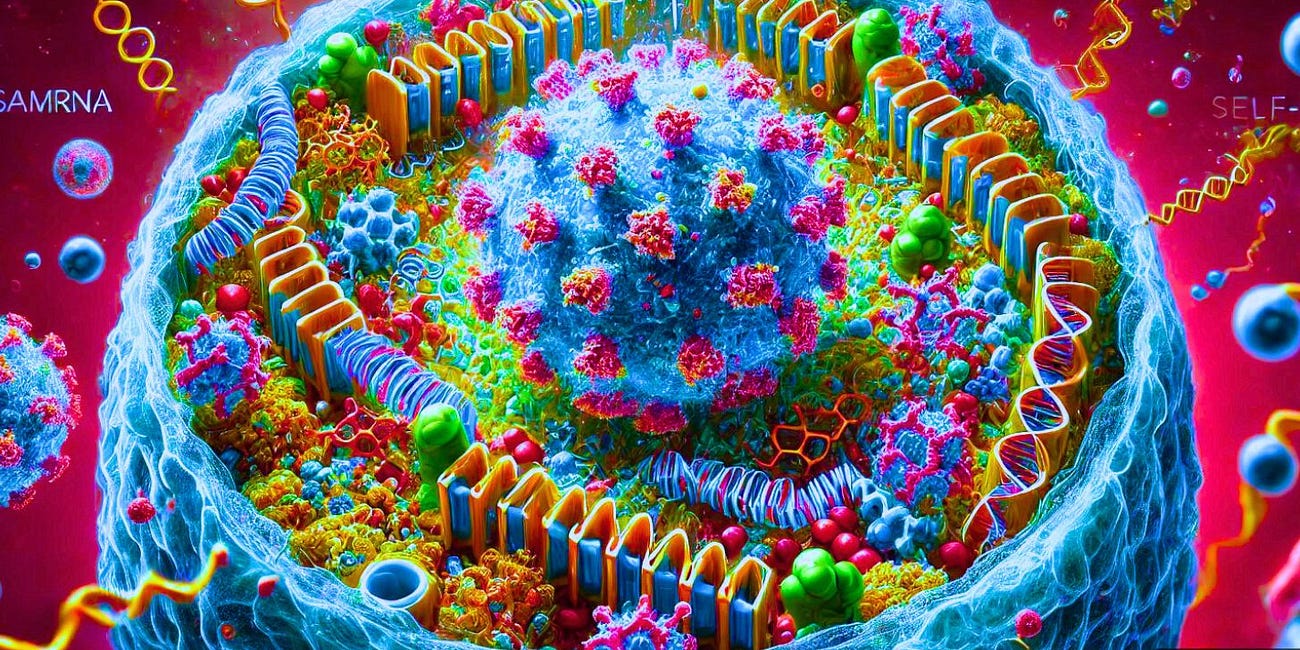
Arcturus Therapeutics, a company specializing in mRNA-based pharmaceuticals, quietly announced Monday that the U.S. Food and Drug Administration (FDA) has granted approval for its Investigational New Drug (IND) application for ARCT-2304, a self-amplifying mRNA (sa-mRNA) injection targeting the H5N1 avian influenza “bird flu” virus.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
The FDA has recently come under fire for “failing to meet safety requirements” and “failing to prioritize scientific data quality delivered from FDA laboratories,” according to the U.S. House of Representatives Committee on Energy and Commerce.
The new Arcturus trial, funded by the Biomedical Advanced Research and Development Authority (BARDA), aims to assess ARCT-2304’s efficacy in preventing pandemic influenza and plans to enroll around 200 healthy adults across the United States.
“Arcturus is actively engaged with the U.S. government to prepare for the next pandemic, and clearance to proceed into the clinic with our STARR® self-amplifying mRNA technology is a key step in this important process,” said Joseph Payne, President & CEO of Arcturus Therapeutics in a press release.
“The Phase 1 clinical trial is designed to evaluate the safety, reactogenicity, and immunogenicity of ARCT-2304 as a potential vaccine to protect against the highly pathogenic H5N1 avian influenza.”
Last month, Arcturus Therapeutics received a nearly $1 million grant from the Bill & Melinda Gates Foundation for “vaccine development,” according to the Foundation’s website.
The grant was given to “improve understanding of durability of protection after administration of nucleic-acid based vaccines.”

In May, this website reported that Bill Gates was funding next-generation mRNA-based bird flu vaccine research in China.
ARCT-2304 is a self-amplifying mRNA drug candidate that uses lipid nanoparticles (LNPs) to deliver genetic material into cells.
Once injected, the vaccine’s mRNA is designed to copy itself within cells, which increases the production of two flu proteins: haemagglutinin (HA) and neuraminidase (NA).
This process purportedly allows for smaller doses compared to typical mRNA vaccines.
The technology is said to be intended to speed up vaccine production in a pandemic and avoid some of the delays of older egg, or cell-based methods.
Additionally, it’s freeze-dried to remain stable in regular refrigerators, potentially simplifying storage and transport—and, by extension, increasing profitability.
Arcturus Therapeutics is owned by BlackRock, a World Economic Forum partner.

This website was the first to raise the alarm about Japan’s rollout of self-replicating jab technology.
As self-replicating mRNA technology is now being rolled out worldwide with little public awareness or scrutiny, questions remain about the safety of these vaccines and whether the global population is prepared—or even informed—about the potential risks involved, as mainstream media largely remains silent on the issue.
Safety Concerns
Utilizing brand-new mRNA-based technology, ARCT-2304 has no long-term safety data.
mRNA jabs are associated with many problems that lead to negative health outcomes, including ingredients like pseudouridine being linked to cancer growth, frameshifting linked to immune system disorders, DNA contamination, and spike protein toxicity.
A January study published in Nature Reviews Drug Discovery confirms there are toxicity risks associated with COVID-19 jabs that use mRNA-based platforms.
In the study, Moderna scientists confirmed that “avoiding unacceptable toxicity with mRNA drugs and vaccines presents challenges” and that “[l]ipid nanoparticle structural components, production methods, route of administration and proteins produced from complexed mRNAs all present toxicity concerns.”
Moreover, safety data from Pfizer Inc. obtained through a Freedom of Information Act (FOIA) request and order of a Texas federal judge confirms COVID jabs using mRNA technology, like those rolled out during the COVID pandemic, are linked to more than 1,200 diseases.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
Bill Gates Should Receive 'Life in Prison Rather Than the More Appropriate Death Sentence for Mass Murder,' Says Candace Owens
In a controversial X (Twitter) exchange, conservative commentator and podcaster Candace Owens made her stance clear on billionaire and vaccine ideologue Bill Gates, urging the Trump campaign to negotiate what she deemed an appropriate punishment for Gates over what she described as “mass murder.”
Bill Gates Funds 'Next-Generation mRNA-Based' Bird Flu Vaccine Research in China Amid H5N1 Pandemic Worries: Journal 'Vaccines'
An October 2023 publication in Vaccines confirms pro-depopulation globalist Bill Gates is funding research into new mRNA vaccines for influenza, amid recent signs of an apparently imminent bird flu pandemic.
Australia Makes $1 Billion Bet On Coming H5N1 Pandemic, Creates Bird Flu Task Force
The Australian government has unveiled a massive $1 billion biosecurity investment to prepare for potential outbreaks of the H5N1 bird flu, a strain it says poses “significant risks” to the nation’s industry, trade, and wildlife.
Japan to Roll Out New Self-Replicating samRNA COVID Shot That Produces Even More Toxic Spike Protein in the Body Than Regular mRNA Jabs
Meiji Seika Pharma announced plans on Tuesday to seek approval in Japan for its self-amplifying mRNA (samRNA) COVID-19 vaccine, Kostaive (ARCT-2301), with a goal of distributing it for the upcoming fall and winter seasons.
Biotech Company Developing Next-Generation Self-Copying 'sa-mRNA' COVID-19 Vaccine Funded by Biden Admin, Bill Gates
Last month, Japan’s Ministry of Health, Labor and Welfare (MHLW) granted approval for ARCT-154, a “self-amplifying mRNA” (sa-mRNA) COVID-19 vaccine for initial vaccination and booster for adults 18 years and older.
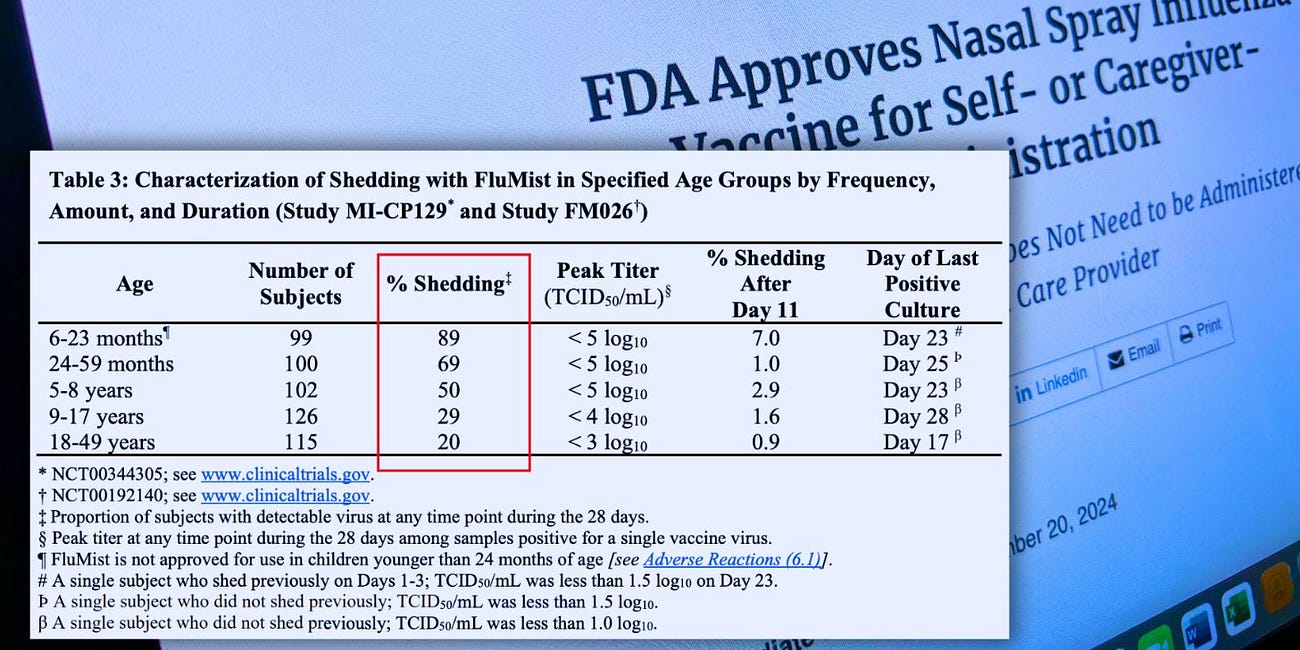
FDA Approves AstraZeneca No-Needle At-Home 'Live' Virus Flu Vaccine with 90% Shed Rate
In September, the U.S. Food and Drug Administration (FDA) approved AstraZeneca’s FluMist for self or caregiver administration for the 2024-2025 influenza season.
FDA 'Failing to Meet Safety Requirements': House Energy & Commerce Committee
In a scathing new report published earlier this month, Energy and Commerce Committee Republicans revealed alarming shortcomings in the Food and Drug Administration’s (FDA) laboratory safety protocols, accusing the agency of failing to meet federal safety requirements critical to protecting public and employee health.
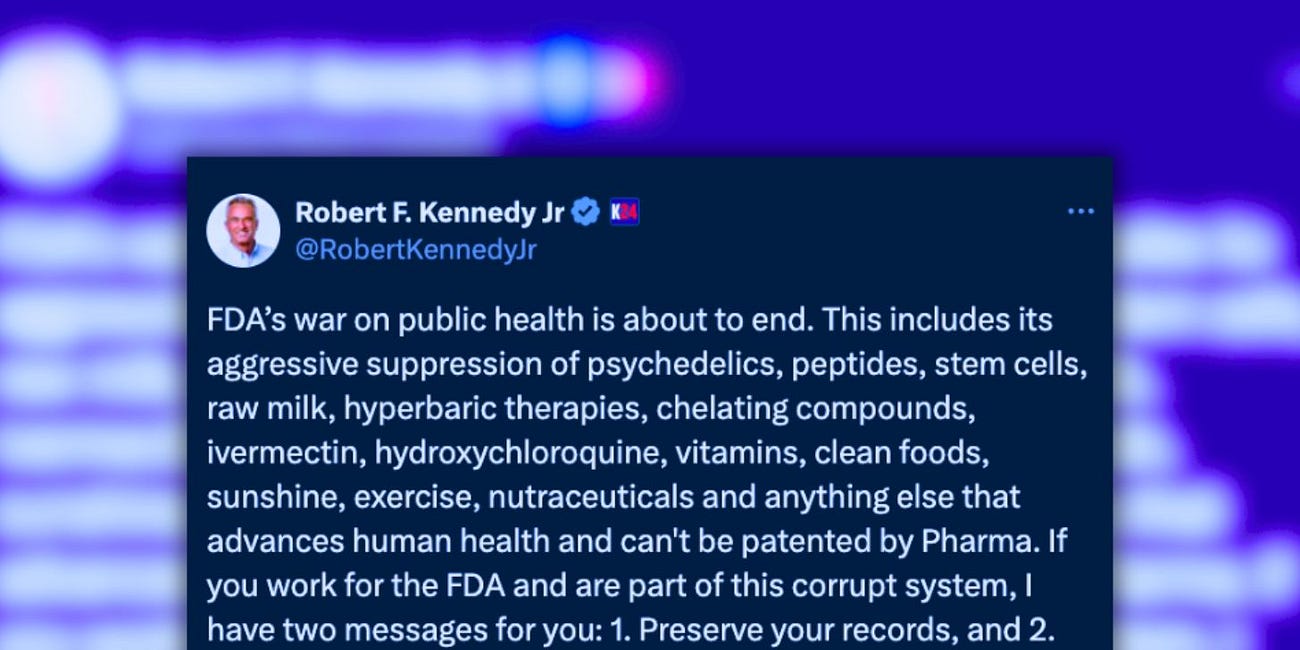
'Preserve Your Records and Pack Your Bags': RFK Jr. Calls Out FDA's 'Corrupt War on Public Health'
On Friday, Robert F. Kennedy Jr. (RFK Jr.) fired a powerful warning shot at the U.S. Food and Drug Administration (FDA), declaring that its “war on public health is about to end.”











Oh no, not you, too? Tamiflu is Gilead's original run-death-is-near. It is a dangerous drug that earlier this year got Emergency Use Instructions to be used in ways that are ineffective and deadly. Please don't promote it or those who are selling it. My worst fear is that Trump, managing the bird flu, will make all the Democrats get vaccinated because they won't believe anything he says and all the Republicans take Tamiflu because they will believe anything he says.
Aren’t these all the same players (bastards) creating the pandemics ?