FDA 'Failing to Meet Safety Requirements': House Energy & Commerce Committee
"FDA is not meeting important federal safety requirements to protect its employees and the public while also failing to prioritize scientific data quality delivered from FDA laboratories," E&C says.
In a scathing new report published earlier this month, Energy and Commerce Committee Republicans revealed alarming shortcomings in the Food and Drug Administration’s (FDA) laboratory safety protocols, accusing the agency of failing to meet federal safety requirements critical to protecting public and employee health.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
The FDA’s stated mission is “promoting and protecting public health,” according to the agency’s website.
However, Committee Chair Cathy McMorris Rodgers (R-WA), along with Health Subcommittee Chair Brett Guthrie (R-KY) and Oversight and Investigations Chair Morgan Griffith (R-VA), issued a letter to Health and Human Services Inspector General Christi Grimm, urging an independent review of the FDA’s Office of Laboratory Safety (OLS) operations.

The irony in this situation is stark: the FDA, an agency entrusted with ensuring public health and safety, particularly through regulating food, drugs, and laboratory practices, is itself failing to follow essential safety protocols.
As the watchdog for national health standards, the FDA is expected to set an example of rigorous adherence to safety regulations to protect both its employees and the public from potential health hazards.
In their letter, House Republicans delivered a stark warning:
“We are deeply concerned that the FDA is not meeting important federal safety requirements to protect its employees and the public while also failing to prioritize scientific data quality delivered from FDA laboratories.”
The statement underscores what lawmakers characterize as significant safety lapses within the FDA, noting that the agency’s alleged failure to uphold safety standards not only endangers staff but compromises the quality and reliability of scientific data emerging from its labs.
The Committee also pointed to chilling examples of oversight deficiencies, including the failure to implement critical recommendations from the Government Accountability Office (GAO) and to comply with federal inspection standards set by the Occupational Safety and Health Administration (OSHA).
By not meeting OSHA’s mandatory inspection requirements, overlooking critical GAO safety recommendations, and failing to support the Office of Laboratory Safety with adequate authority and resources, the FDA is breaching the very standards it demands of others.
This contradiction undermines public trust, as it suggests that the agency responsible for safeguarding health and enforcing high safety standards in laboratories cannot maintain those standards within its own operations.
You can read E&C’s full letter here:
Longstanding GAO Recommendations Unmet
In a striking revelation, the Committee’s letter highlighted the FDA’s ongoing non-compliance with GAO recommendations dating back to 2020.
Following an investigative report by the GAO, five essential recommendations were issued to the FDA, advising the agency to address systemic issues, clarify roles and responsibilities, and ensure OLS had sufficient authority and resources for effective laboratory safety oversight.
Despite commitments from FDA Commissioner Robert Califf to fulfill these recommendations, the Committee reported that “none of the five recommendations have been fully addressed.”
The report paints a troubling picture of an agency that appears unwilling or unable to address safety protocols, despite clear mandates from both GAO and congressional oversight bodies.
Non-Compliance with OSHA Safety Standards and Inadequate Inspections
According to the letter, the FDA has fallen critically short of OSHA’s annual inspection requirements, essential for maintaining lab safety.
While OSHA mandates yearly inspections for laboratories, the FDA reportedly follows a “rotating basis” for inspecting only a third of its labs annually.
Independent OLS inspections occur every three years rather than yearly, leaving two-thirds of FDA labs unsupervised by OLS during any given year.
This limited oversight model has yielded glaring discrepancies between OLS findings and internal FDA lab inspections.
For example, OLS inspections “make significantly more findings than the FDA center inspections.”
During a 2023 OLS inspection of a Center for Drug Evaluation and Research (CDER) facility, 19 non-conforming findings were recorded, contrasting starkly with the center’s own inspections, which reported only one finding in 2020 and none in 2021.
These discrepancies suggest that the current inspection approach may be insufficient to identify and address safety risks effectively.
Realignment of Lab Safety Oversight Raises Transparency Concerns
The Committee letter further condemned the FDA’s 2018 realignment of the Office of Laboratory Safety, alleging a lack of transparency and documentation.
In an unexplained move reportedly based on a McKinsey consulting assessment, the FDA transferred oversight of OLS back to the Office of Chief Scientist (OCS) without notifying Congress or producing documentation substantiating the decision.
This reorganization reversed a previous structure designed to ensure direct accountability, moving OLS oversight out of the Commissioner’s purview and potentially undermining OLS’s ability to enforce lab safety protocols.
“There was no documented justification for the realignment and renaming of OLS,” the letter noted, adding that the FDA’s refusal to produce records to Congress has raised serious questions about the agency’s intentions.
Systemic Deficiencies in FDA Safety Standards
The Committee’s findings paint a grim picture of the FDA’s internal safety oversight.
Its report emphasizes systemic failures, highlighting how these lapses not only jeopardize the safety of FDA personnel but also raise doubts about the quality and reliability of scientific research from its labs.
As an agency tasked with protecting public health, the FDA’s alleged disregard for safety protocols raises critical questions about its priorities and operational transparency.
The Committee has requested that Inspector General Grimm and the Office of Evaluations and Inspections (OEI) conduct an exhaustive review of the FDA’s support for OLS, focusing on whether it is sufficiently funded, independently operated, and given the authority necessary to enforce compliance with federal safety regulations.
This investigation into the FDA’s safety practices comes as confidence in government oversight of health agencies is increasingly scrutinized.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
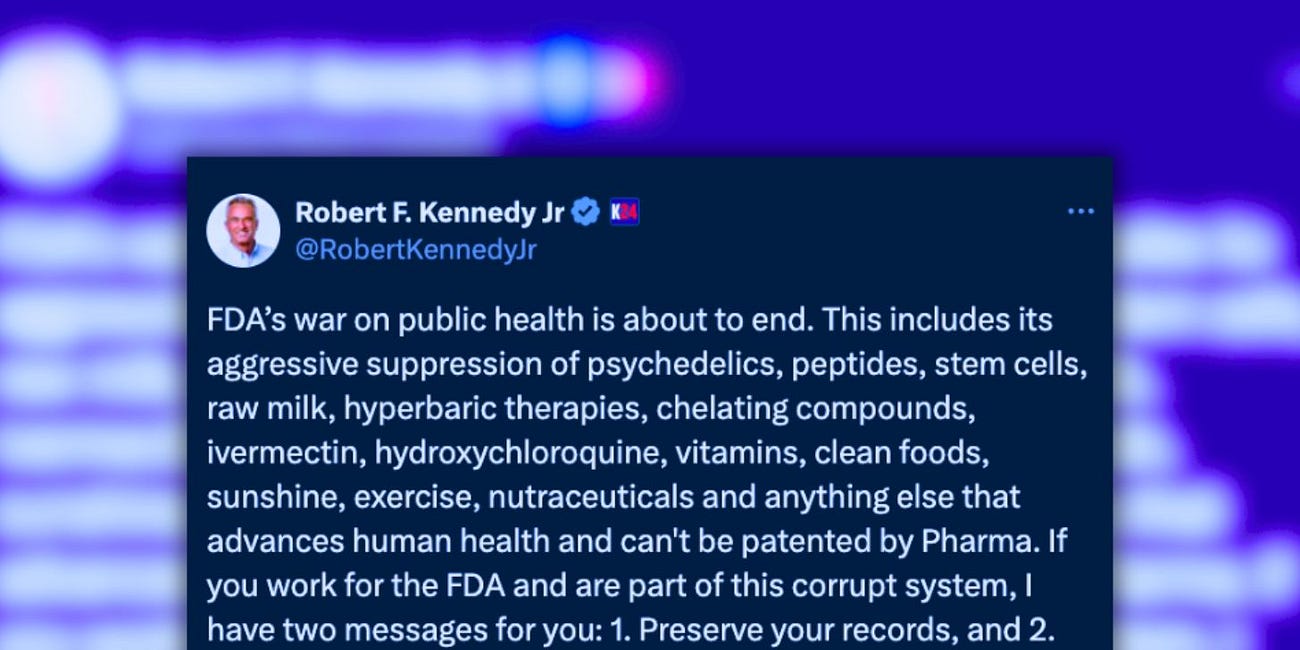
'Preserve Your Records and Pack Your Bags': RFK Jr. Calls Out FDA's 'Corrupt War on Public Health'
On Friday, Robert F. Kennedy Jr. (RFK Jr.) fired a powerful warning shot at the U.S. Food and Drug Administration (FDA), declaring that its “war on public health is about to end.”
FDA to Skip Drug Approval Process for Bird Flu Vaccine: Dr. Peter Marks Confirms Agency Will Leverage Controversial 'Emergency Use Authorization' (EUA) Tactic as It Did with Deadly COVID Jab
Dr. Peter Marks, Director of the Center for Biologics Evaluation and Research (CBER) at the U.S. Food and Drug Administration (FDA), confirmed last week that the FDA will skip the rigorous drug approval process for influenza bird flu (H5N1) vaccines, as it did for COVID-19 jabs.
U.S. Army, FDA, CDC Refuse to Cooperate with Florida's COVID-19 Vaccine Safety and Efficacy Investigation
A Florida grand jury, convened by Republican Governor Ron DeSantis to investigate the efficacy of COVID-19 vaccines, revealed on Friday that several federal agencies have declined to cooperate with their inquiries.







The FDA works for Satan, not Americans. Their goal is not happy or healthy for Americans.
They are traitors, as are the other Governmental agencies and the cia fbi...drain the swamp...