Tumor-Linked 'Bovaer' Methane-Reducing Cattle Feed Supplement Used in U.S. Is Excreted in Cow's Milk: New Zealand Environmental Protection Authority
FDA admits Bovaer "is a drug" but classifies it as "feed ingredient," skipping more rigorous drug approval process.
In a significant study assessing the safety and metabolism of the methane-reducing feed additive Bovaer® (3-NOP or DSM073738) in lactating dairy cows, researchers found that a substantial portion of the compound and its metabolites are excreted into milk.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
Nevertheless, Elanco Animal Health Incorporated—owned by BlackRock and Vanguard—in May announced the U.S. Food and Drug Administration (FDA) had completed its review of Bovaer, a “first-in-class methane-reducing feed ingredient, and determined the product meets safety and efficacy requirements for use in lactating dairy cattle.”
As of July, 150 U.S. farms have enrolled on Elanco’s Uplook online tool that tracks dairies using Bovaer.
It was announced last winter that the U.S. Department of Agriculture gave Dairy Farmers of America (DFA) $22.8 million to incentivize dairies in California, Utah, and Idaho to use Bovaer.
The announcement indicated the drug was already being used in cows in Colorado, New Mexico, Kansas, Vermont, and New York.
Elanco, who can be contacted here, has received significant financial support from the Bill & Melinda Gates Foundation.
A letter from the FDA to the Director of Global Nutritional Health Regulatory, Dr. G. Allen Bridges, confirms that while Bovaer “is a drug,” the agency chose to “refrain” from putting the drug through the more rigorous animal drug approval process, instead classifying it simply as a feed supplement.
The FDA animal drug approval process is more comprehensive and rigorous than the process for animal feed supplements, requiring extensive safety and efficacy testing, longer review periods, and stricter regulatory oversight compared to the simpler, often self-determined safety assessments for many feed supplements.
The agency’s decision to classify a substance they admit is a drug as a feed additive raises significant safety concerns and questions about the agency’s motives.
The FDA letter also confirms Bovaer is “[n]ot for human use” because it “may damage male fertility and reproductive organs, is potentially harmful when inhaled, and is a skin and eye irritant.”
Moreover, Bovaer is toxic and has been linked to tumor growth.
Components of Bovaer are also excreted in the cow’s milk we drink.
This discovery raises important questions about residues in milk from treated cows and their potential implications for human consumption.
A comprehensive analysis completed by New Zealand’s Environmental Protection Authority (EPA) confirms a carcinogenicity study found “tumours in female rats which could be due to 3-NOP treatment and to demonstrate carcinogenic potential.”
Dr. Richard Bartlett told this website that the FDA’s disregard for safety and regulatory requirements on a new cattle drug with unknown long-term effects highlights the need for reform under new leadership to protect public health.
“The current FDA has gone rogue,” Dr. Bartlett said. “In my opinion, it is utterly irresponsible for the FDA to arbitrarily bypass requirements for new animal drug approval, pharmaceutical current Good Manufacturing Practices, adverse event reporting, and proper labeling of a new drug with no long-term safety evidence.”
“This drug, intended for use in cattle, will ultimately be consumed by humans. New federal healthcare public servants, under a new HHS Secretary Kennedy, will work to reduce health risks for all Americans,” the 30-year Texas ER doctor added.
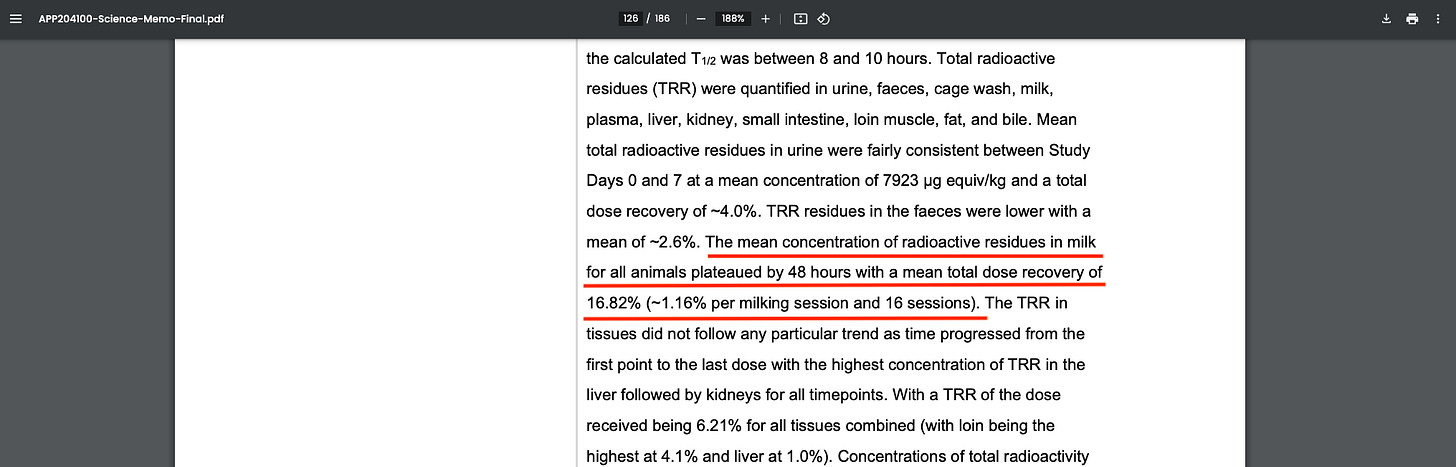
New Zealand EPA Confirms Over 16% of Bovaer Dose Found in Milk, Raising Consumer Safety Concerns
The New Zealand EPA document also confirms 16.82% of the administered dose of Bovaer was excreted via cows’ milk during the study period.
This makes milk the largest excretion pathway for the compound, compared to urine (4%) and feces (2.6%).
Researchers used radioactive labeling of DSM073738 to trace the compound and its breakdown products, even in minute quantities.
These findings suggest that Bovaer and its byproducts become part of the milk’s biochemical makeup, raising questions about the long-term effects of such residues on humans consuming dairy products.
While Bovaer’s primary stated goal is to reduce methane emissions from dairy cows the findings from this study highlight potential unintended consequences.
The incorporation of metabolites into milk sugars and the excretion of radioactive residues in dairy products raises questions about the long-term safety of milk from treated animals.
As Bovaer moves closer to widespread use, public health advocates and regulatory agencies must ensure that its environmental benefits do not come at the cost of consumer safety.
The presence of Bovaer metabolites in milk represents a critical area for further investigation and public discourse.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
Widespread Tissue Accumulation of Bovaer: High Residue Levels Found in Key Organs
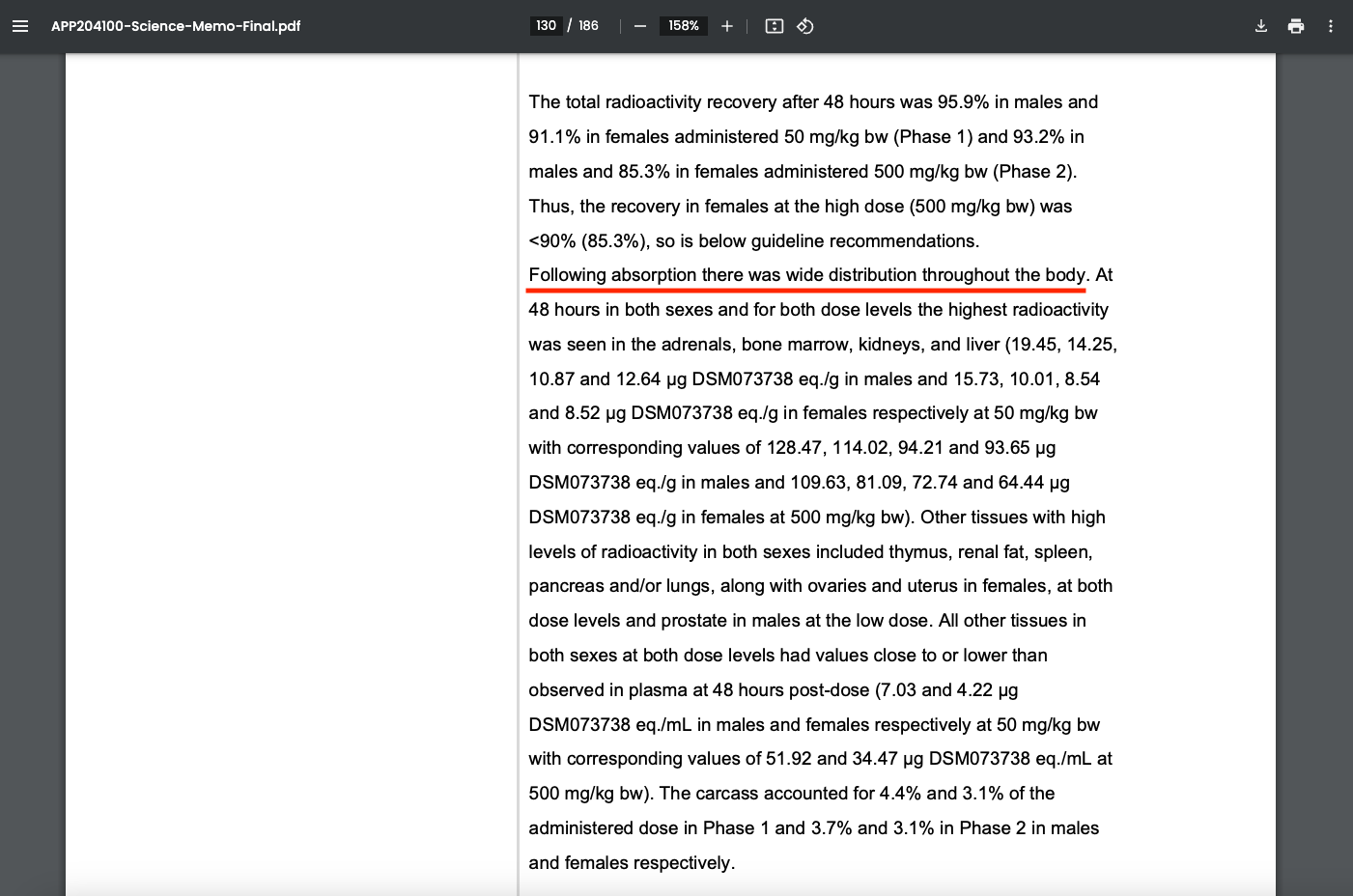
The New Zealand study revealed that DSM073738 (Bovaer) and its associated radioactive residues were widely distributed throughout the body, with notable accumulation in specific organs and tissues.
The highest levels of radioactivity were found in the adrenal glands, bone marrow, kidneys, and liver, indicating these as primary sites of retention.
Additional significant accumulation was observed in the thymus, renal fat (fat surrounding the kidneys), spleen, pancreas, and lungs.
Sex-specific tissues also exhibited radioactivity, with females showing notable concentrations in the ovaries and uterus, while males exhibited accumulation in the prostate at the lower dose (50 mg/kg).
Residual radioactivity was also detected in the carcass, accounting for a small percentage of the administered dose (4.4% in males and 3.1% in females at the low dose; 3.7% in males, and 3.1% in females at the high dose).
Additionally, measurable levels were found in plasma, with concentrations varying by dose and sex.
This distribution demonstrates extensive tissue penetration of DSM073738, particularly in metabolic, excretory, and endocrine-related organs.
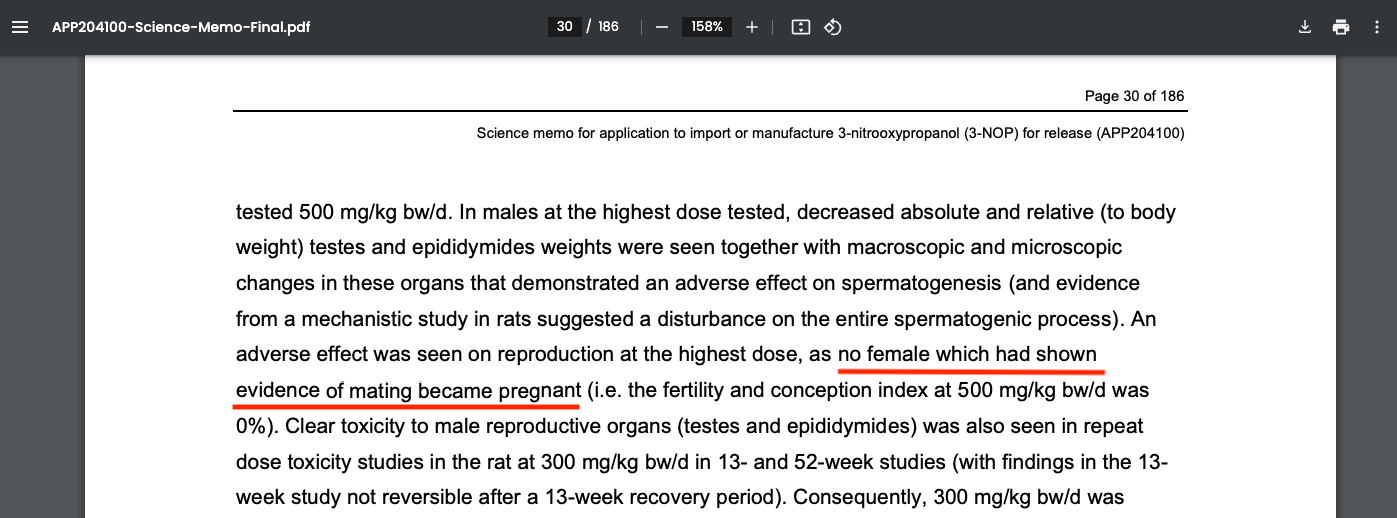
No Mating Female Rats Became Pregnant After Exposure to Bovaer
Female rates who had shown evidence of mating were unable to become pregnant after exposure to the drug.
Gastrointestinal Toxicity
During post-mortem examination of an animal sacrificed at 2,000 mg/kg bw, dark red foci were discovered in the jejunum and ileum (sections of the small intestine).
These dark red foci likely indicate hemorrhagic lesions or localized damage to the intestinal tissue.
This suggests that high doses of 3-NOP may cause severe gastrointestinal toxicity, possibly due to irritation or internal bleeding.
The presence of a drug classified as a feed additive in milk, coupled with evidence of carcinogenicity, tissue accumulation, and reproductive toxicity, demands urgent independent scrutiny—not just for the safety of the dairy supply but to expose the troubling regulatory gaps allowing such risks to reach consumers unchecked.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
'Past 485 Million Years' of Climate Data Confirm Earth Coolest It's Ever Been: Journal 'Science,' Washington Post
A brand new study published Thursday in the journal Science confirms that we live in the coolest time it’s ever been on Earth over the last “485 million years.”
Sun Drives Earth's Climate, Not CO2: 30-Page Study in Journal 'Geomatics' Refutes Mainstream Climate Change Narrative
A groundbreaking new study published Tuesday in the peer-reviewed journal Geomatics challenges the prevailing narrative surrounding the causes of so-called “climate change.”
'Climate Researchers' Want to Block the Sun by Injecting Aerosols Into the Sky: MIT Review
A February publication in MIT Technology Review explains that researchers have been working on injecting particles into the stratosphere to reflect sunlight for a long time.
Human CO2 Emissions 'Hardly Discernible in Observational Data,' Play 'Minor Role' in Climatic Evolution: Journal 'Sci'
A study published earlier this year in Sci, an international, peer-reviewed, open-access journal published quarterly online by MDPI, confirms humans have made no discernable impact on the long-term isotopic signature of carbon in the atmosphere.
WEF-Aligned United Nations at Center of International Efforts to Block the Sun with Chemicals Sprayed Into Sky
Switzerland has submitted a proposal to create the first United Nations (UN) “expert group” to examine the use of controversial ‘solar radiation management’ (SRM), which involves blocking sunlight from reaching Earth.
Gates-Funded Technology Sprays Concoction from Aircraft Carrier Into Sky to Block the Sun in California: 'Extraordinarily Dangerous' Solar Radiation Modification (SRM)
In Alameda, California, a team of scientists from the University of Washington is testing so-called marine cloud brightening, a controversial geoengineering technique purportedly to cool Earth in the name of “climate change.”
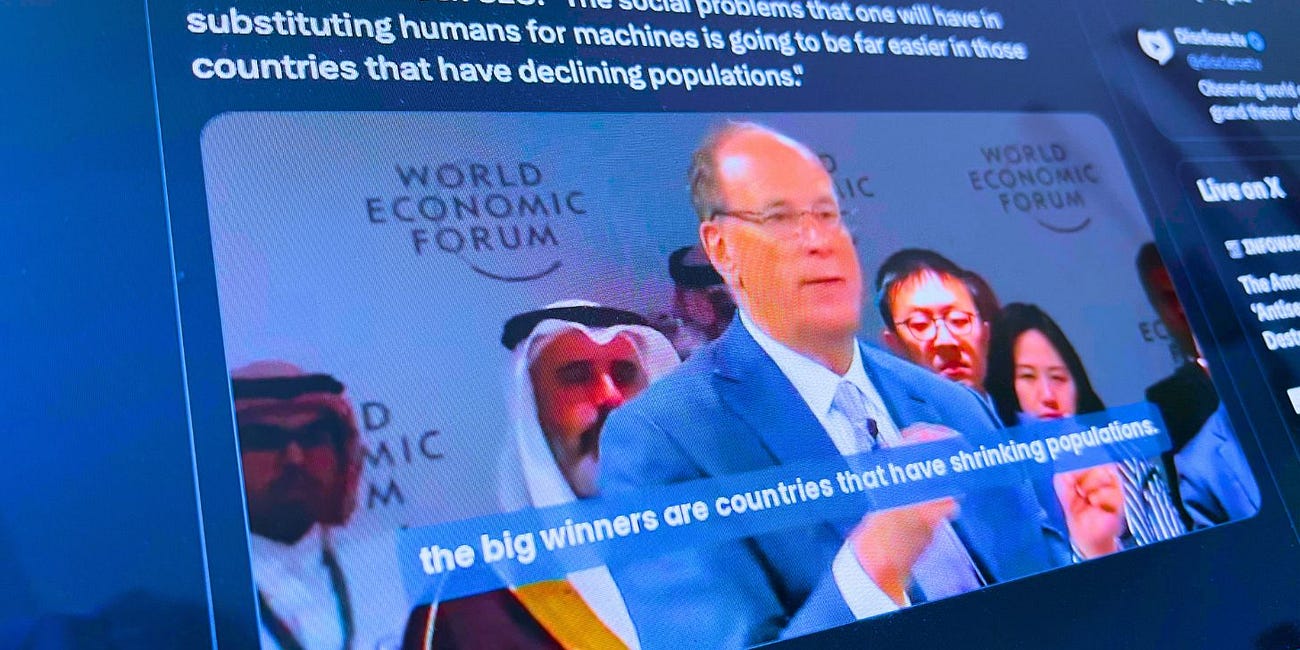
Depopulation Agenda Endorsed by WEF-Allied BlackRock CEO Larry Fink: 'Shrinking Populations' Better for 'Robotics and AI' Takeover (Video)
BlackRock Inc. CEO Larry Fink praised depopulation, an idea associated with reducing the number of people in a country or region, in remarks made during the World Economic Forum’s (WEF) recently held ‘Special Meeting on Global Collaboration, Growth and Energy Development’ in Saudi Arabia.
FDA Greenlights New Bill Gates-Funded ARCT-2304 Self-Replicating samRNA 'Pandemic' H5N1 Bird Flu Jab
Arcturus Therapeutics, a company specializing in mRNA-based pharmaceuticals, quietly announced Monday that the U.S. Food and Drug Administration (FDA) has granted approval for its Investigational New Drug (IND) application for ARCT-2304, a self-amplifying mRNA (sa-mRNA) injection targeting the H5N1 avian influenza “bird flu” virus.















They are poisoning humanity. Thank you for this write up.
Thank you, Jon, for compiling and sharing this important expose. I've added a link to your article into my collection of Bovaer resources in my "red pill" library:
> Library: BeyondC19.org
> Bovaer: https://workflowy.com/s/beyond-covid-19/SoQPdY75WJteLUYx#/14f6462cefdd