FDA Informed Consent Waiver Allows Your Blood, Genetic Data, and Personal Health Information to Be Taken Without Your Knowledge
Rule has been effective since January 2024.
In a move that raises significant ethical and privacy concerns, the U.S. Food and Drug Administration (FDA) in January 2024 finalized a rule permitting Institutional Review Boards (IRBs) to waive or alter informed consent requirements for clinical investigations deemed “minimal risk.”
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
An IRB, like the one at Johns Hopkins University (heavily funded by the Bill & Melinda Gates Foundation), reviews and approves research studies for clinical trials involving humans.
Effective since January 22, 2024 and buried in regulatory jargon, the FDA’s waiver rule effectively grants pharmaceutical companies, government agencies, and other organizations the ability to access and use Americans’ private health data, including blood and genetic material, without their consent or knowledge.
The implications are staggering.
Under this waiver, any person’s health data, biospecimens (such as blood or tissue samples), or medical records can be accessed and used for FDA-regulated research if it meets vague and highly subjective criteria.
The rule summary reads:
The Food and Drug Administration (FDA, the Agency, or we) is issuing a final rule to amend its regulations to implement a provision of the 21st Century Cures Act (Cures Act). This final rule allows an exception from the requirement to obtain informed consent when a clinical investigation poses no more than minimal risk to the human subject and includes appropriate safeguards to protect the rights, safety, and welfare of human subjects. The final rule permits an institutional review board (IRB) to waive or alter certain informed consent elements or to waive the requirement to obtain informed consent, under limited conditions, for certain FDA-regulated minimal risk clinical investigations.
You can download the full document below:
FDA Authorizes Use of Private Health Data and Biospecimens Without Consent
One of the most alarming aspects is that the waiver allows researchers to use data or biospecimens from individuals who may never know they are part of a study.
The FDA explicitly acknowledges this: “This final rule allows an exception from the requirement to obtain informed consent when a clinical investigation poses no more than minimal risk to the human subject and includes appropriate safeguards to protect the rights, safety, and welfare of human subjects.”
But who defines “appropriate safeguards,” and are they enough?
The FDA claims this rule is necessary for advancing research, but the fine print tells a different story.
According to the FDA’s own language, the waiver allows access to identifiable private information or biospecimens.
The document states, “If the clinical investigation involves using identifiable private information or identifiable biospecimens, the clinical investigation could not practicably be carried out without using such information or biospecimens in an identifiable format.”
In plain terms, your name, medical history, blood samples, or genetic information could be taken and used without your knowledge.
Subjectivity in Minimal Risk Definition Opens the Door to Abuse
The definition of “minimal risk” further muddies the waters.
The rule defines it as “the probability and magnitude of harm or discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests.”
This subjective benchmark opens the door to broad interpretations, leaving the public at the mercy of IRBs with varying standards and oversight.
FDA Dismisses Public Trust Concerns
Public trust in medical research is already at a low point following the COVID-19 pandemic, and this rule threatens to erode it further.
The FDA brushes off concerns about public perception, noting, “Several comments argue that waivers of informed consent weaken human subject protections and would allow IRBs to retreat from their human subject protection responsibilities.”
Yet, instead of addressing these concerns, the agency doubles down, stating, “We believe that the rule upholds the principles underlying existing ethical standards.”
The FDA’s dismissal of additional safeguards is particularly troubling.
Critics recommended a centralized registry to track individuals included in these studies to prevent overuse or misuse of the waiver.
The FDA refused, arguing, “Such a registry might present additional risks regarding privacy and confidentiality of participant data.”
Ironically, the FDA’s solution to privacy concerns is to grant unfettered access to health data in the first place.
IRBs Operate with Questionable Oversight
The oversight responsibility lies with IRBs, which are tasked with approving these waivers.
But IRBs themselves operate under questionable transparency and consistency.
The FDA acknowledges the risks: “Some comments express concern that IRBs might inappropriately grant waivers for clinical investigations that are greater than minimal risk, or that they may fail to appreciate both the nature and risks of procedures in the research studies that are submitted to them for review.”
Big Pharma and Government Can Access Your Biospecimens
Adding to the concern, organizations conducting clinical investigations can utilize this waiver, meaning not only academic institutions and healthcare systems but also for-profit pharmaceutical companies and contract research organizations (CROs).
This includes entities like Pfizer, Moderna, or even government agencies like the CDC.
The broad language apparently applies to any FDA-regulated clinical investigation.
Since pharmaceutical companies routinely conduct such investigations for product development or regulatory approval, they are included among the entities that could access the data.
These groups can now tap into health records, blood samples, and genetic data without notifying or obtaining consent from the individuals involved.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
Your Genetic Data Could Be Weaponized Against You
The most chilling potential consequence of this rule is how nefarious actors could use your genetic information to make a bioweapon specific to the individual.
Genetic data collected through research could fall into the hands of foreign adversaries or malicious entities.
For example, many Americans have already given their DNA information to private companies like 23andMe.
The genetic testing company says 14 million people have taken a 23andMe DNA test.
Suzanne Bernstein, a law fellow at the Electronic Privacy Information Center, said: “There are no serious safeguards, no regulation around the collection and sale of really sensitive personal data. For 23andMe, the nefarious [data] breach constitutes a security issue, but so does the company sharing your information with a party that you didn’t know about. Customers may technically consent to their data being shared by accepting the terms and conditions, but those are really long and a lot of people don’t read them.”
U.S. Representative Jason Crow (D-CO), a member of the House Intelligence Committee, warned in July 2022, “You can’t have a discussion about this without talking about privacy and the protection of commercial data.”
“The CCP [Chinese Communist Party] will undoubtedly use the genetic data collected by [entities like] BGI to further its malign aggression, potentially even to develop a bioweapon used to target the American people,” he added.
Weaponizing genetic data isn’t theoretical.
According to a bipartisan congressional effort in the BIOSECURE Act, entities like the Beijing Genomics Institute (BGI) have collected genomic data from Americans and worked with the Chinese military.
This data could be leveraged to develop biological weapons targeting individuals or specific populations.
Such technology could allow adversaries to create precision bioweapons capable of incapacitating or killing based on genetic markers.
Even within the U.S., there is the potential for misuse by unethical actors.
Data gathered under the guise of research could be repurposed to discriminate, surveil, or exploit individuals, undermining civil liberties and national security.
Without robust safeguards, this rule leaves the door wide open for abuses of power and malicious intent.
A Dangerous Shift in Consent Protections
This rule represents a seismic shift in how informed consent is handled in clinical investigations.
By allowing waivers for accessing identifiable private information and biospecimens, the FDA has opened the floodgates for the widespread use of Americans’ health data, including blood and genetic material, without their permission.
The public must demand greater transparency, accountability, and protections to ensure that our rights to privacy and autonomy are not sacrificed in the name of research expediency.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
FDA 'Failing to Meet Safety Requirements': House Energy & Commerce Committee
In a scathing new report published earlier this month, Energy and Commerce Committee Republicans revealed alarming shortcomings in the Food and Drug Administration’s (FDA) laboratory safety protocols, accusing the agency of failing to meet federal safety requirements critical to protecting public and employee health.
FDA Greenlights New Bill Gates-Funded ARCT-2304 Self-Replicating samRNA 'Pandemic' H5N1 Bird Flu Jab
Arcturus Therapeutics, a company specializing in mRNA-based pharmaceuticals, quietly announced Monday that the U.S. Food and Drug Administration (FDA) has granted approval for its Investigational New Drug (IND) application for ARCT-2304, a self-amplifying mRNA (sa-mRNA) injection targeting the H5N1 avian influenza “bird flu” virus.
FDA 'Fast Tracks' 2 Combination COVID-19-Influenza Shots Containing Formaldehyde, Insect DNA, Toxic Detergent Banned in Europe: Bill Gates-Funded Manufacturer
The U.S. Food and Drug Administration has granted “Fast Track designation” for two Sanofi combination vaccine candidates for influenza an…
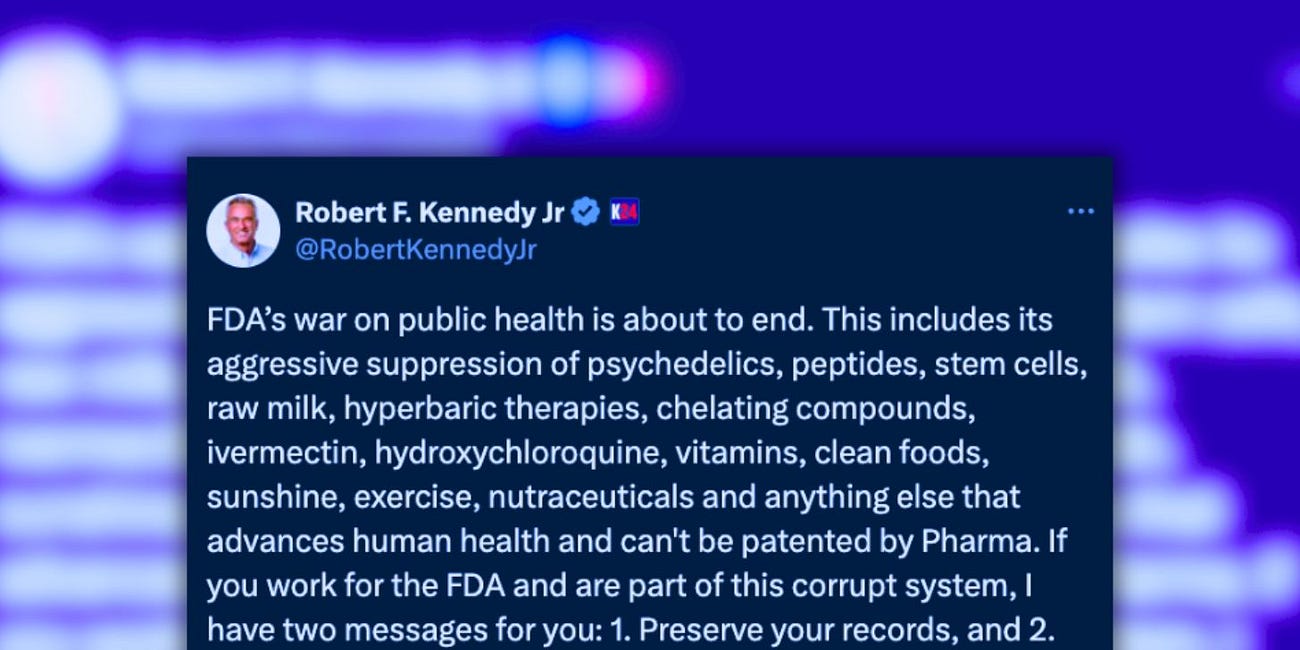
'Preserve Your Records and Pack Your Bags': RFK Jr. Calls Out FDA's 'Corrupt War on Public Health'
On Friday, Robert F. Kennedy Jr. (RFK Jr.) fired a powerful warning shot at the U.S. Food and Drug Administration (FDA), declaring that its “war on public health is about to end.”
Aaron Siri Issues Legal Notice to HHS, Demands Preservation of Records, Threatens DOJ Referral
Aaron Siri, a prominent attorney who has represented figures like Robert F. Kennedy Jr., has sent an official legal notice to the U.S. Department of Health and Human Services (HHS) and its subsidiaries, including the CDC, FDA, NIH, CMS, and others, warning against the destruction, deletion, or modification of any records.
U.S. Defunded EcoHealth for Gain-of-Function Oversight Lapses Only to Waive It for 'Any Federal Agency' Conducting Deadly Pathogen Experiments: Bioweapons Wild West?
In a striking sequence of events, the U.S. government defunded EcoHealth Alliance on May 15, 2024, for cited gain-of-function oversight lapses, only to waive gain-of-function oversight requirements for every single federal agency just days earlier.















Altho perhaps only made “official” of recent “daze,” the ability to take ALL of our info, health or otherwise has been happening. Ever look at how they manage your pictures on your eye phone, even following children’s faces through the years & grouping them together w/o even being asked to provide this “service.” And then there are willing victims who submit to 23 & Me, etc. Nothing is private any more, unless you totally unplug, avoid all conventional medical & hospitals & become Amish or a Bushman or such.
PS-An overpriced “Wellness” kit of which some contain controversial ingredients.
Without your knowledge, pretty much sums it up across the board