Europe Authorizes 'Powder' Self-Amplifying mRNA COVID-19 Injection 'Kostaive' Made by Bill Gates-Funded Arcturus Therapeutics
European Medicines Agency greenlights samRNA COVID shot, joining U.S. and Japan in novel technology rollout.
Last week, the Committee for Medicinal Products for Human Use (CHMP) quietly recommended granting marketing authorization for the new self-amplifying mRNA (samRNA) COVID-19 shot ‘Kostaive’ (ATC code: J07BN01) made by Arcturus Therapeutics.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
CHMP is the European Medicines Agency (EMA) committee responsible for evaluating and authorizing human medicines in the European Union (EU).
The EMA is roughly equivalent to the U.S. Food and Drug Administration (FDA).
What Are Self-Amplifying Vaccines?
Self-amplifying vaccines use RNA technology engineered to replicate within cells, raising concerns about uncontrolled antigen production, prolonged exposure to spike protein, long-term risks to the immune system, and other health risks.
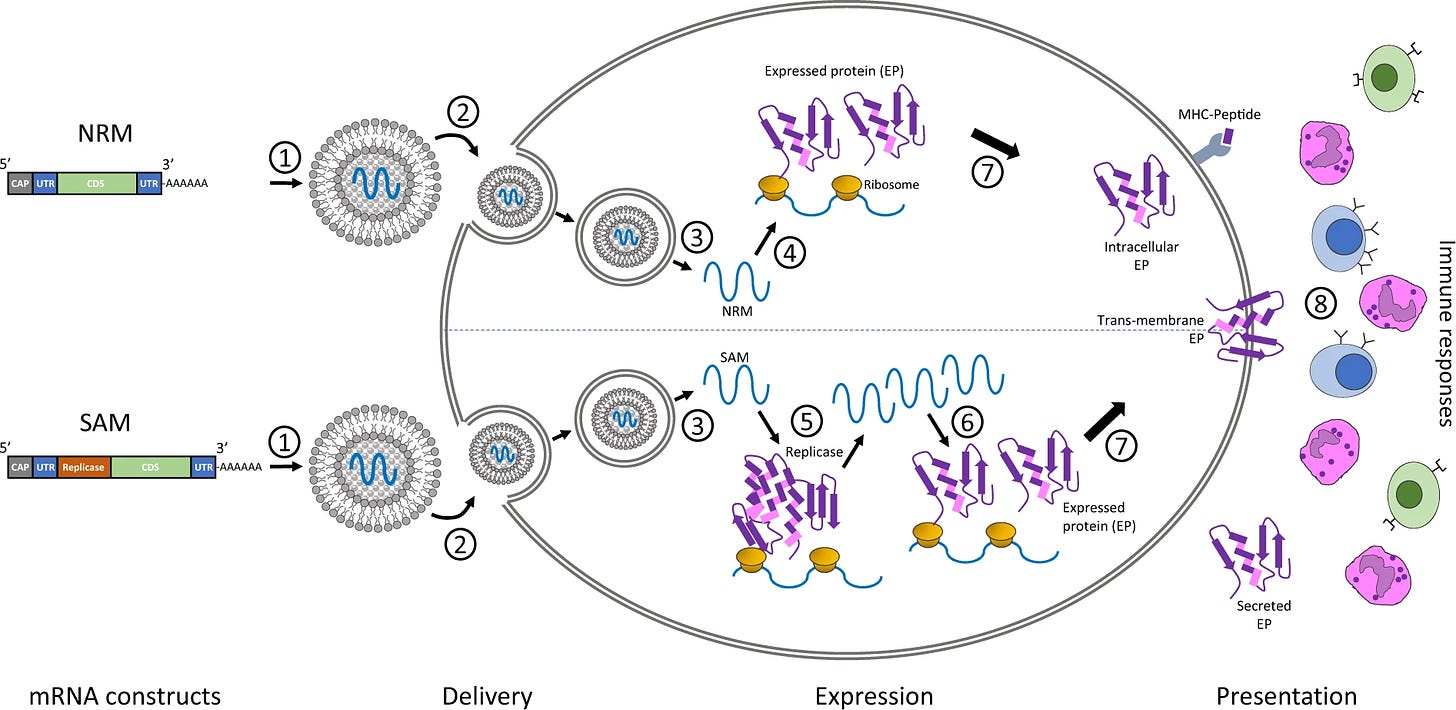
Regular mRNA vaccines, such as Pfizer’s COVID-19 jab, are said to instruct human cells to produce spike protein for a limited time.
However, self-replicating mRNA vaccines not only force the body to produce the spike protein within cells but also include mechanisms that make the RNA replicate itself inside the cell, amplifying the production of spike protein over an extended period.
mRNA vaccines have been associated with a range of issues that contribute to negative health outcomes, including links to over 1,200 diseases, pseudouridine tied to cancer growth, waning immunity, breakthrough infections, frameshifting tied to immune system disorders, contamination with foreign DNA and the SV40 cancer gene sequence, ingredient toxicity, spike protein toxicity, and death.
Both Japan and the United States have already begun efforts to roll out various Arcturus samRNA products.
Kostaive samRNA Available in ‘Powder’ Form: Aerosolization?
“On 12 December 2024, the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, recommending the granting of a marketing authorisation for the medicinal product Kostaive, a vaccine intended for the prevention of COVID-19 in adults,” a CHMP press release reads.
The announcement reveals Kostaive will not only be available for injection, but also in “powder” form.
“Kostaive will be available as a powder for dispersion for injection,” it says.
The powdered form of Kostaive is apparently designed for reconstitution before injection but could theoretically be adapted for aerosolization, raising potential concerns about environmental exposure and unintended distribution.
Bill Gates, BlackRock, & WEF Ties
In October, Arcturus Therapeutics received a nearly $1 million grant from the Bill & Melinda Gates Foundation for “vaccine development,” according to the Foundation’s website.
The following month, the FDA granted approval for Arcturus Therapeutics’ ARCT-2304, an H5N1 avian influenza “bird flu” samRNA injection.
Now Europe, along with the U.S. and Japan, will be deploying this technology apparently with no long-term safety testing.
Arcturus Therapeutics is owned by BlackRock, a World Economic Forum partner.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood