1 in 34 AstraZeneca COVID Jab Recipients Suffer Serious Adverse Event (SAE): European Union Clinical Trial Data
Vaccinated more than twice as likely to suffer SAE than those who received placebo.
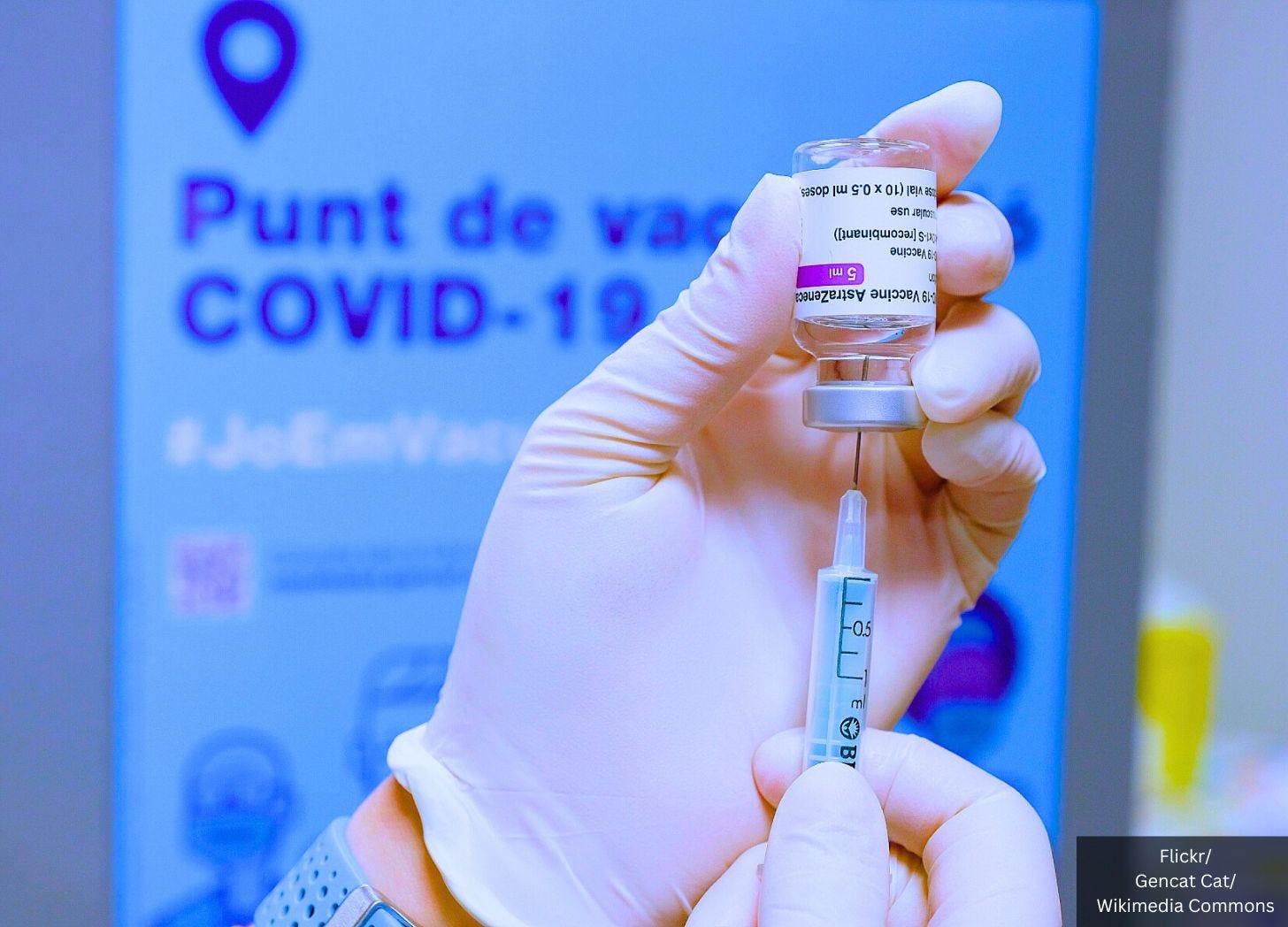
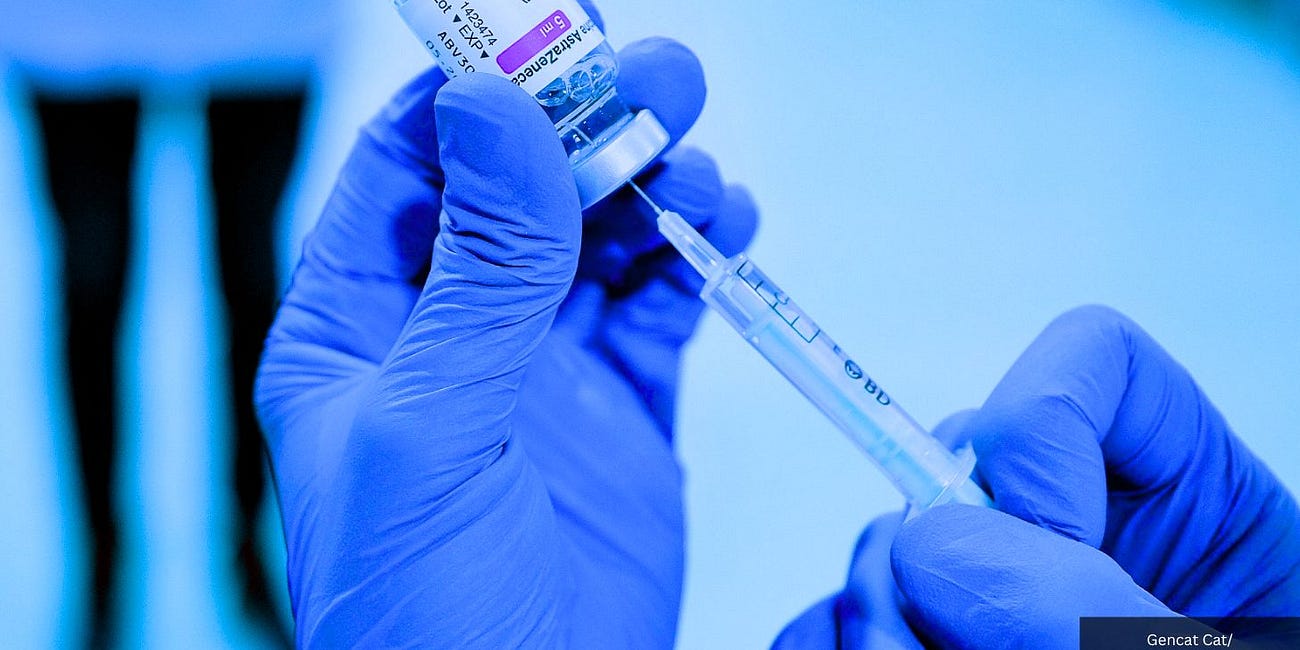
Clinical trial data submitted to the European Union Clinical Trials Register confirms one in 34 recipients of AstraZeneca Plc.’s COVID-19 injection suffer a serious adverse event (SAE).
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
The troubling revelation comes after AstraZeneca withdrew the marketing authorization for its COVID shot in the European Union (EU).
The pharmaceutical giant has also been confronting multiple claims from individuals who allege injuries and deaths arising from the company’s vaccine.
AstraZeneca admitted in court that its COVID jab can cause thrombosis with thrombocytopenia syndrome (TTS), a rare condition characterized by blood clots and low levels of platelets following vaccination.
The new clinical trial documentation defines an SAE as fulfilling one or more of the following criteria: “death; immediately life-threatening; in-participant hospitalization or prolongation of existing hospitalization; persistent or significant disability or incapacity; congenital abnormality or birth defect; an important medical event.”
The trial represented a phase 3, double-blind, placebo-controlled, multicenter study conducted at 88 sites across the United States, Peru, and Chile.
It lasted from August 2020 to March 2023 and enrolled 32,449 participants aged 18 years or older who were healthy or had stable chronic medical conditions.
Participants were randomized 2:1 to receive either two doses of AstraZeneca’s AZD1222 vaccine or placebo (saline) given 4 weeks apart.
The primary objectives were to evaluate the safety and efficacy of AZD1222 in preventing symptomatic COVID.
The trial was sponsored by AstraZeneca and funded by BARDA, the U.S. Department of Defense, the National Institute of Allergy and Infectious Diseases (NIAID), and the National Institutes of Health (NIH).
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
The data show that 621 out of 21,587 (one in 34) vaccine recipients suffered an SAE.
That’s compared to just 136 out of 10,793 (one in 79) in the placebo group, meaning the vaccinated were more than twice as likely to suffer a serious adverse event.

The data also indicate that 4,750 (one in four) vaccine recipients suffered medically attended adverse events (MAAEs), defined as adverse events “leading to medically-attended visits that were not routine visits for physical examination or vaccination, such as an emergency room visit, or an otherwise unscheduled visit to or from medical personnel (medical doctor) for any reason.”
That’s compared to just 1,256 (one in eight) in the placebo group.
Moreover, 2,516 (one in eight) vaccine recipients suffered adverse events of special interest (AESIs), defined as “events of scientific and medical interest specific to the further understanding of the study intervention safety profile and required close monitoring and rapid communication by the investigators to the sponsor.”
Only 591 (one in 18) in the placebo group experienced an AESI.
The clinical trial results were pointed out by the U.K.’s Dr. David Cartland, a General Medical Council (GMC)-registered practitioner, who provided a link to the data in a Tuesday X (formerly Twitter) post.
“Needs to go viral!” Dr. Cartland wrote. “This is absolute proof of the vaccine failure. Astra Zeneca quietly submitted their Clinical Trail data as part of their application to have their license withdrawn.”
“No MSM outlet has reported on this,” he rightly pointed out.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
European Union Withdraws AstraZeneca COVID-19 Jab Authorization
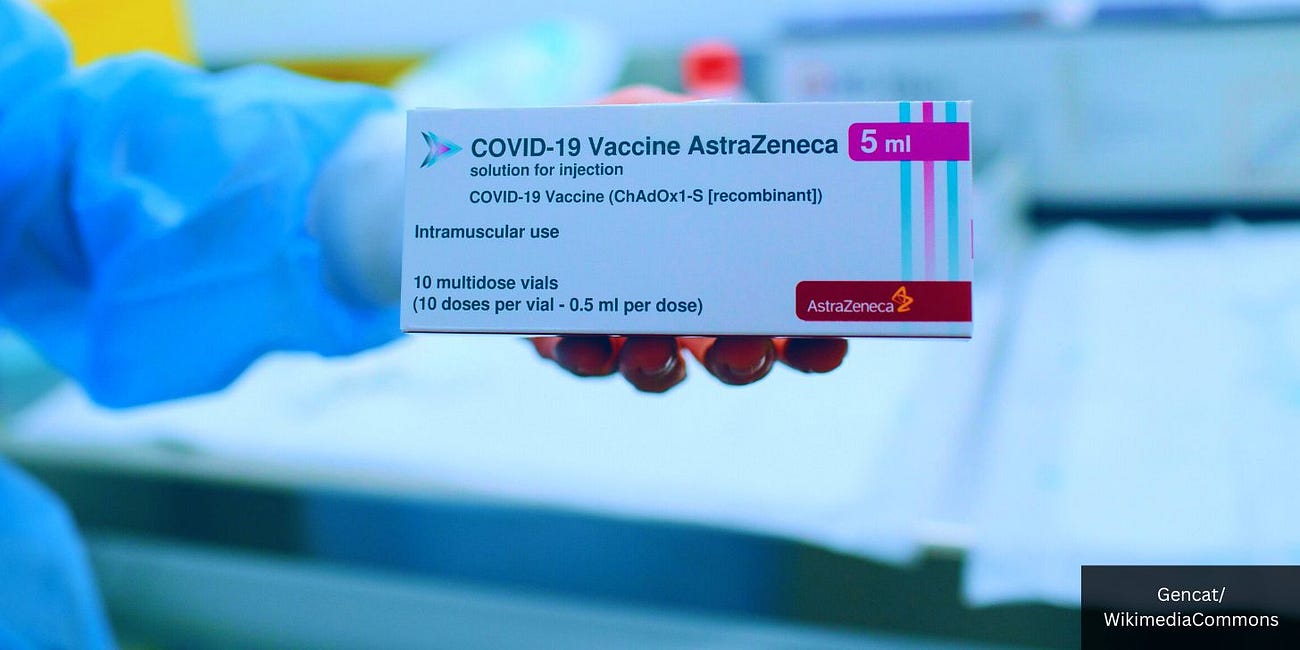
The European Union’s (EU) European Commission has announced the withdrawal of the marketing authorization for the “Vaxzevria” COVID-19 vaccine, developed by AstraZeneca Plc., upon the company’s request.
AstraZeneca Admits Its COVID Jab Can 'Cause' Blood Clots for First Time in Court Documents
The pharmaceutical manufacturer AstraZeneca is being sued in class action over claims its viral vector COVID-19 jab caused death and serious injury in dozens of cases, The Telegraph reports.
AstraZeneca Faces £80M Compensation for U.K. Vaccine Injury/Death Claims
In what is shaping up to be a monumental legal battle, AstraZeneca is confronting claims from 35 individuals who allege complications arising from the AstraZeneca COVID-19 DNA vaccine. AstraZeneca is an official partner of the World Economic Forum (WEF) and
'Disease X Act of 2023' Introduced to Develop New 'Medical Countermeasures for Viral Threats with Pandemic Potential' Through BARDA Agency that Funded COVID Jabs: U.S. House of Representatives
A bill for developing new medical interventions against a potential future “pandemic” was quietly introduced to the U.S. House of Representatives on June 5, 2023.
Trump-Era Executive Order 13887 Authorized U.S. Defense Department to 'Facilitate Development of Next-Generation Influenza Vaccines' Just 3 Months Before COVID Pandemic
President Donald J. Trump signed Executive Order 13887 on September 19, 2019, just months before the COVID-19 pandemic began and a novel mRNA vaccine would be developed for the disease.








It would be interesting to also find out what they used as the non-vaccine (supposed placebo) as according to information AstraZenica provided to the TGA in Australia (still available on TGAs website) they used Meningococcal vaccine as the “placebo” 😳.. which on its own could increase adverse events in this group to make the overall percentage difference less..
Even with this mass information. The heathens already have vaccines with mrna ready too go. For the overhyped bird flu. The USA and EU want too vaccinate the farmers. In other words get rid of them. Just like the chickens,Cows etc. There overall agenda is too be able too control the food therefore control the population.