Nearly 1 in 10 COVID Patients Treated Early with Remdesivir Die—Late Treatment Raises ICU, Oxygen Therapy Risks: Central European Journal of Medicine
"Patients who received remdesivir on day 4 or later after first symptoms had a 2-3 time higher chance to need high flow oxygen therapy and a more than 4-fold higher risk to be admitted to the ICU."
A new study published Monday in the peer-reviewed medical journal Wiener Klinische Wochenschrift (in English, “Vienna Clinical Weekly”; subtitled The Central European Journal of Medicine), confirms that administration of remdesivir after day three in hospitalized COVID-19 patients increases risk of ICU admission and need for high flow oxygen therapy.
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
Remdesivir (brand name Veklury), manufactured by biopharmaceutical company Gilead Sciences, is an antiviral medication approved for treating COVID in adults and children.
It’s one of the three antivirals the U.S. Food & Drug Administration (FDA) recommends for treating the disease, and was pushed heavily by the mainstream media throughout the pandemic.
However, the new study conducted by Austrian researchers shows remdesivir worsens patient outcomes when used in late treatment for COVID.
“Compared to patients who received remdesivir within the first 3 days after symptom onset, administering remdesivir after day 3 in hospitalized COVID-19 patients is associated with higher risk for complications, such as the need for high-flow oxygen therapy and ICU admission,” the study reads.
The authors looked at 219 patients: 148 (67.6%) in the early treatment group and 71 (32.4%) in the late group.
Most of the patients (68.9%) were vaccinated.
“Late remdesivir administration was associated with a significantly higher probability of needing high-flow oxygen therapy (OR 2.52, 95% CI 1.40-4.52, p = 0.002) and ICU admission (OR 4.34, 95% CI 1.38-13.67, p = 0.012) after adjusting for confounders,” the authors write.
“In the late group there was a trend towards a higher risk of clinical worsening (OR 2.13, 95% CI 0.98-4.64, p = 0.056) and need for any oxygen therapy (OR 1.85, 95% CI 0.94-3.64, p = 0.074).”
While the study claimed that early remdesivir administration (0–3 days since symptom onset) “may” decrease the risk of COVID complications, the majority of patient deaths occurred in those who received the drug early.
Alarmingly, nearly one in 10 (8.8%) patients in the early remdesivir treatment group died, a more than three-fold increase compared to the late treatment group.
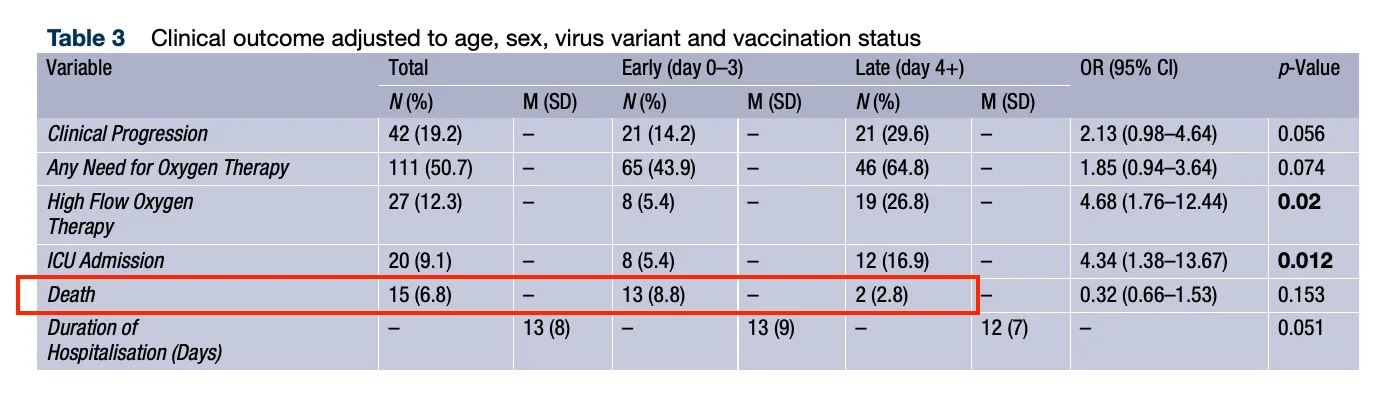
And more than four in 10 (43.9%) patients who took remdesivir early required oxygen therapy.
But the late group especially “showed a trend towards an increased probability of clinical deterioration (OR 2.13 95% CI 0.98 to 4.64, p= 0.056) and any need for oxygen therapy.”
“Patients in the late group had a significant higher probability of receiving high-flow oxygen therapy (OR 2.52 95% CI 1.40 to 4.52, p= 0.002) or being admitted to ICU (OR 4.34 95% CI 1.38 to 13.67, p=0.012), after adjusting for age, sex, vaccination status, and viral variant,” the authors write.
“Patients who received remdesivir on day 4 or later after first symptoms had a 2–3 time higher chance to need high flow oxygen therapy and a more than 4-fold higher risk to be admitted to the ICU. Furthermore, the risk for clinical progression seemed to have doubled in patients who received remdesivir after day 4 of symptom onset and there was a trend for an increased need for any oxygen therapy.”
Significantly, the study had no control group to represent patients who did not receive remdesivir.
The authors admit this “restricts the conclusions to the comparison between early and late remdesivir administration, making it challenging to evaluate remdesivir’s overall efficacy in COVID-19 treatment.”
You can download the full study here:
Follow Jon Fleetwood: Instagram @realjonfleetwood / Twitter @JonMFleetwood / Facebook @realjonfleetwood
Throughout the pandemic, safe, effective, and relatively inexpensive drugs for treating COVID, such as budesonide and ivermectin (which can be purchased together here at 10% off when using code ‘FLEETWOOD’ at checkout), were demonized by health authorities and the mainstream media.
Ivermectin has been approved by the FDA for decades.
Though the FDA does not advocate for ivermectin’s use to treat COVID, the agency recently agreed in response to a lawsuit to delete social media posts it made demonizing the drug.
Moreover, a study published in April in the Iranian Jundishapur Journal Of Health Sciences confirmed that ivermectin is a safe and effective treatment for COVID.
The study authors found ivermectin reduced the length of intensive care unit (ICU) admission, decreased the symptomatic period for most acute symptoms, and did so without producing any negative side effects.
“The findings demonstrated that ivermectin significantly reduced the need for Intensive Care Unit admission (32.7% vs. 5.5%; P < 0.001), hospitalization duration (six vs. four days; P < 0.001), and median time to symptom resolution period (P < 0.05) in COVID-19 patients compared to the placebo group, without any serious side effects (P > 0.05),” the authors write.
They emphasized that “[a]mong those administered ivermectin, there were no reports of sensitivity reactions, adverse effects, or drug-related toxicity.”
Regarding budesonide, Oxford University’s dual STOIC and PRINCIPLE trials decisively confirmed budesonide’s safety and effectiveness against COVID.
The studies showed that when given to adults with early COVID, budesonide “reduced the likelihood of requiring urgent care, emergency department consultation, or hospitalisation.”
There was also a “quicker resolution of fever, a known poor prognostic marker in COVID-19, and self-reported and questionnaire-reported symptom resolution was faster. There were fewer participants with persistent COVID-19 symptoms at days 14 and 28 after budesonide therapy compared with usual care.”
Inhaled budesonide “improves time to recovery, with a chance of also reducing hospital admissions or deaths (although our results did not meet the superiority threshold), in people with COVID-19 in the community who are at higher risk of complications.”
Altogether, questions are raised as to why remdesivir was and continues to be promoted by so-called health authorities and mainstream media outlets when budesonide and ivermectin appear to be cheaper, safer, and more effective treatments for COVID.
'Ivermectin Is an Effective Medication' for COVID-19: Double-Blind, Randomized Clinical Trial: 'Jundishapur Journal of Health Sciences'
A new study published last month in the Iranian Jundishapur Journal Of Health Sciences confirms that the antiviral drug ivermectin is a safe and effective treatment for COVID-19.
'Medicine That Could Save Your Dying Son's Life': Dr. Bartlett Advocates for Patient Rights, Life-Saving Treatment in Ohio Senate Testimony (Video)
On Wednesday, emergency room director Dr. Richard Bartlett, MD, appeared before the Ohio Senate Health Committee to testify in favor of a piece of pro-patient rights legislation that would allow citizens of the state to freely collaborate with their doctor and choose medical treatment options they believe are right for them.
Thousands of Doctors, Hundreds of Political Figures Demand COVID Shots Pulled Off Market, Pledge to Deny Big Pharma Donations: Dr. Mary Talley Bowden with 'Americans for Health Freedom'
Firebrand otolaryngologist Dr. Mary Talley Bowden recently revealed that thousands of doctors and hundreds of elected and campaigning political figures have signed a public pledge affirming that COVID-19 shots are not safe and effective.
Do Hospital Profits or 'We The People' Run Ohio's Legislature? Medical Freedom Bill Delayed by Healthcare Industry-Funded Senate Chair
In a series of steps toward safeguarding patient healthcare liberties, the ‘Dave and Angie Patient and Health Provider Protection Act,’ House Bill 73 (HB 73), has been making significant headway through Ohio’s legislative channels, spearheaded by Republican State Representatives










They used Remdesivir to kill my mom in early 2022. 😢
I’m part of a big class action lawsuit although I wonder if it’ll ever go anywhere. 🙏🏼