FDA-Approved Drugs Nitazoxanide (NTZ) and Monoclonal Antibodies 'Inhibit' Ebola
Concerns about an Ebola outbreak follow news Denver healthcare workers are being vaccinated with a "shedding" live-virus Ebola vaccine.
Healthcare workers at Denver Health in Colorado were recently injected with a “live” Ebola vaccine that has a 31% “shed” rate, according to the U.S. Food & Drug Administration (FDA) insert.
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood
When a vaccine sheds, the vaccinated individual could infect unsuspecting bystanders.
The FDA package insert for the ‘ERVEBO’ Ebola vaccine states that the drug “shed[s]” onto others 31.7% of the time and for up to 20 days after vaccination.
At the same time, a new $12 million, U.S.-taxpayer-funded bat lab that will reportedly potentially study Ebola is being built in Fort Collins, Colorado, just 65 miles north of Denver Health.
This is despite Fort Collins’ already checkered safety record handling the deadliest pathogens on earth.
These reports have raised questions about which readily available medicines are safe and effective for treating Ebola infection.
Nitazoxanide (NTZ) and monoclonal antibodies represent two such treatments.
In fact, NTZ is reported to be effective against both DNA and RNA viruses, which represent all viruses.
Dr. Richard Bartlett is a 30-year Texas emergency room (ER) physician and recipient of the Meritorious Service Award from the Texas Department of Health and Human Services for his numerous contributions to improving the health of all Texans.
Dr. Bartlett is calling for more studies into the safety and effectiveness of NTZ against all potential pandemic viruses.
“We need institutions to do more studies to assess whether NTZ is safe and effective for treating any and all future potential pandemic viruses,” the former advisor to Texas Governor Rick Perry’s Health Disparities Task Force says.
Bartlett discussed the Colorado Ebola predicament with Republican Texas Representative Ronny Jackson.
Rep. Jackson reminded Bartlett of another situation in California, involving an illegally-run Chinese laboratory that “posed a threat to public health for allegedly conducting dangerous research related to COVID-19,” according to a U.S. House Committee On Oversight and Accountability press release.
The Texas representative indicated Donald J. Trump would be the best 2024 presidential choice for successfully dealing with these challenges.
Iowa Senator Joni Ernst (R) also weighed in on the matter.
“We cannot allow any batty experiments of pandemic potential to be unleashed on our own shores,” she said. “Americans have suffered enough from Fauci-funded risky research, which is why I am working to defund EcoHealth that funneled taxpayer dollars to the Chinese state-run Wuhan Lab.”
“The world cannot afford another lab leak, especially one on U.S. soil or near our military bases.”
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood
Nitazoxanide (NTZ)
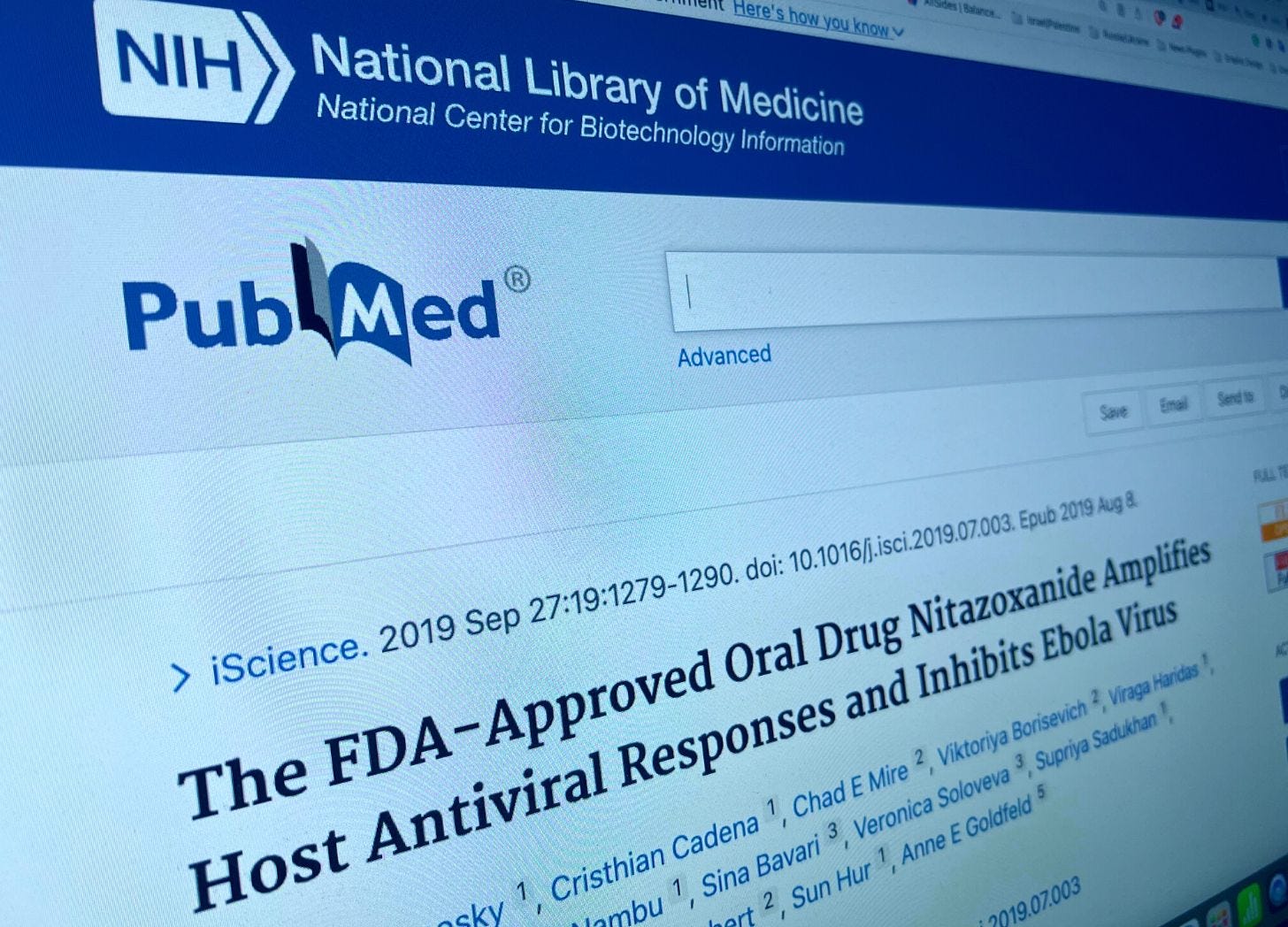
A 2019 publication in iScience confirms the FDA-approved oral drug NTZ “broadly amplifies the host innate immune response to viruses and inhibits Ebola virus (EBOV) replication.”
Boston Children’s Hospital later praised the study, emphasizing how the drug “enhances the immune system’s ability to detect Ebola, normally impeded by the virus.”
A March 2022 publication in Viruses also cited the iScience study, emphasizing how the paper showed NTZ “could broadly enhance innate antiviral immunity, which inhibited EBOV replication and counteracted EBOV VP35’s immune suppression.”
Monoclonal Antibodies
A December 2019 publication from the U.S. National Institutes of Health (NIH) reported how two monoclonal antibodies—Ridgeback Biotherapeutics’ mAb114 (Ansuvimab; Ebanga) and Regeneron’s REGN-EB3 (Inmazeb)—reduced the risk of death from Ebola.
Both monoclonal antibodies are FDA-approved (here; here).
In August 2022, the World Health Organization (WHO) published guidelines for Ebola virus disease therapeutics, with strong recommendations for use of the same two monoclonal antibodies, mAb114 and REGN-EB3.
In March 2023, a study published in Frontiers confirmed the safety and efficacy of Ebanga.
Dr. Bartlett, who helped with the establishment of a Regeneron monoclonal antibody infusion center in Odessa that treated COVID-19 patients from all over Texas, has seen the effectiveness of Regeneron’s infusion firsthand.
“I saw patients drastically improve during the thirty-minute monoclonal antibody infusion,” he said. “I worked side by side with nurses. We saw patients with shortness of breath, chest pain, fatigue, and headaches become symptom-free within that thirty-minute infusion.”
“During the first month, the regional hospitals reported a precipitous drop in COVID patients. We were emptying the hospitals because of our Regeneron infusion center.”
The ER doctor went on to describe how the Biden Administration attempted to thwart that success.
“Tragically, in the middle of the COVID pandemic, President Joe Biden arbitrarily cut the distribution of Regeneron monoclonal antibodies to Texas and Florida by 50%,” Bartlett lamented. “I’m still speechless Biden did that in the middle of a pandemic.”
“If there is a future pandemic, I am convinced we would have better leadership under President Trump than Joe Biden.”
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood




“which is why I am working to defund EcoHealth that funneled taxpayer dollars to the Chinese state-run Wuhan Lab.”
Shouldn’t an activity which is illegal to do in the USA (GOF Research)also be illegal to fund such activity regardless of where it is done.
https://rumble.com/v489fih-breaking-hospitals-injecting-staff-with-live-ebola-vaccine.html