Systemic Autoinflammatory Disease Following COVID-19 Vaccination: Journal 'Cureus'
'Still's disease' was listed as an adverse event on Pfizer's safety data before the jab was made available to the public.
A case report published last month in the peer-reviewed journal Cureus reveals the development of adult-onset Still’s disease (AOSD) following mRNA COVID-19 vaccination.
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood
AOSD is an autoinflammatory disorder, belonging to the category of inflammatory arthritis.
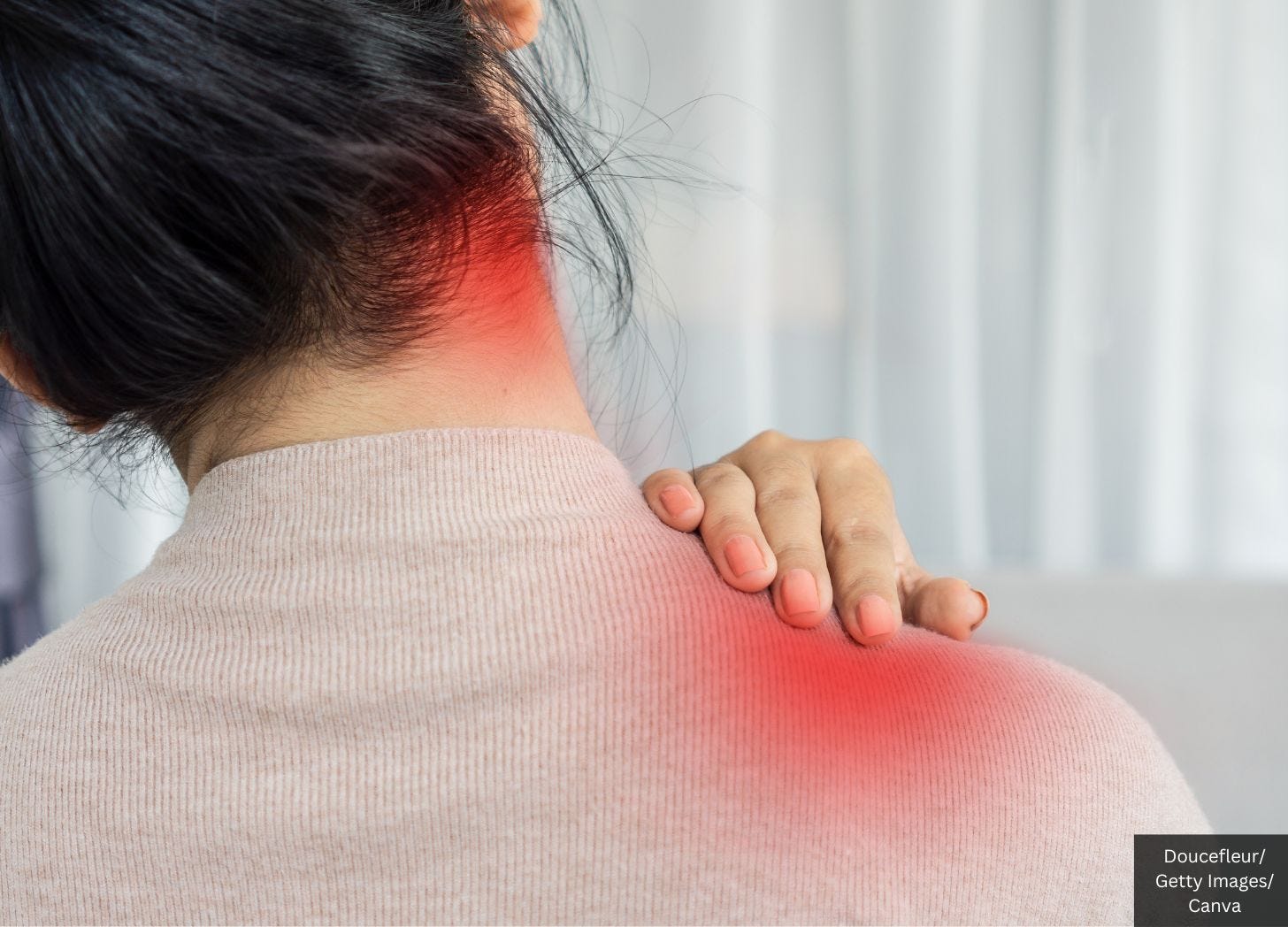
The condition manifests through symptoms including elevated fever, skin rash, and joint discomfort.
Although new-onset disease at >80 years of age is uncommon, the case report authors present a case of an 82-year-old woman with AOSD that developed after receiving a COVID shot.
“AOSD mostly affects young adults with the first peak between 15 and 25 years old and the second at 36 to 46 years old, and its new onset at over 80 years is uncommon,” they confirm. “[T]his case demonstrates that AOSD can be induced by COVID-19 vaccination even in older individuals.”
The authors also explained that coronavirus jabs can cause inflammation and immune-related problems: “COVID-19 vaccines are known to cause overproduction of cytokines, systemic inflammation, and some immune-mediated adverse events, such as rheumatoid arthritis, systemic lupus erythematosus, dermatomyositis, vasculitis, and polymyalgia rheumatica after the vaccination has been reported.”
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood
They emphasized there have been dozens of cases of AOSD following COVID vaccination and that the period from vaccination to the disease onset varies from 1 day to 4 months following the first or second dose.
The woman was referred to the hospital due to a 20-day history of high-grade fever, erythema (redness of the skin), and arthralgia ( joint pain) at the wrists that developed the “day after receiving the fifth dose of mRNA-based vaccine (BNT162b2, Pfizer) for COVID-19.”
Pfizer Inc.’s safety data—only made available by order of a Texas federal judge—show the company and the U.S. Food & Drug Administration (FDA) were aware that Still’s disease was linked to the shot before making it available to the public.

The woman had visited a local clinic and was prescribed antipyretics and antibiotics at first, but the symptoms “did not improve.”
Physical examination revealed skin redness on her neck chest wall, and thighs.
Her wrist joints were swollen, red, and tender, and her fingers were swollen.
The authors warn that AOSD should be considered for patients with persistent fever and rash, even in older patients, and that research is needed to understand the link between COVID-19 vaccines and AOSD.
“When patients present with persistent fever and rash, clinicians should consider AOSD as a possible differential diagnosis even in older individuals,” the authors conclude. “It will be necessary to study a number of cases to clarify the risk of COVID-19 vaccines regarding AOSD development.”
The authors are affiliated with the Department of General Internal Medicine at Kobe City Medical Center General Hospital in Hyogo, Japan.
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood




Yep. 61 year old male today. All his specialists treating their pertinent symptoms. Not one sees big picture. Baffled Im sure.