Skin Condition Pityriasis Rosea 'Following COVID-19 Vaccinations': 'Oman Medical Journal'
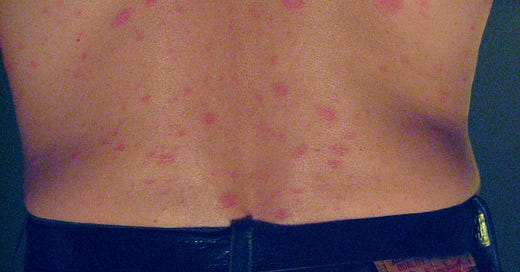
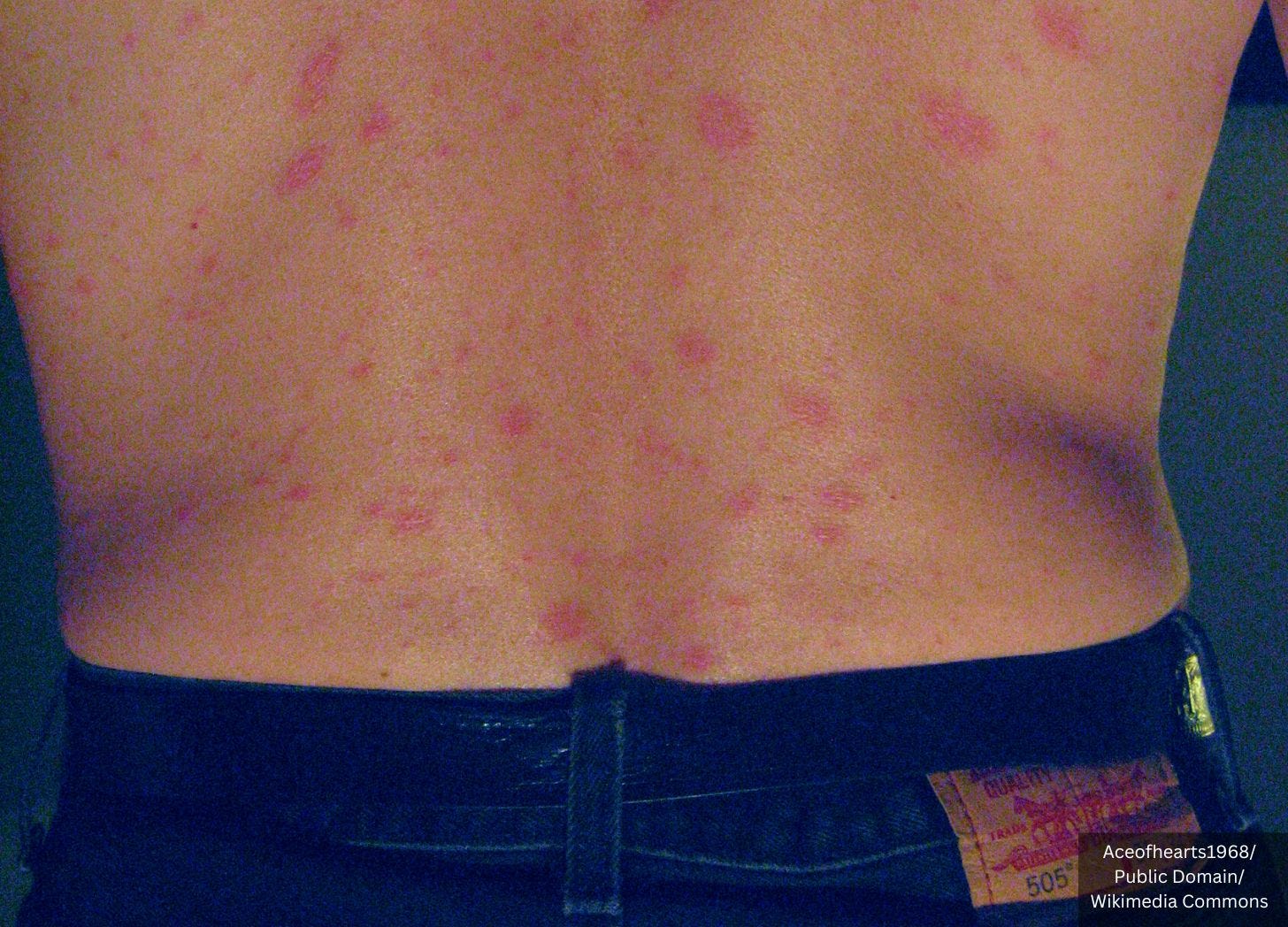
Pityriasis rosea is a skin condition with a big round or oval "herald patch" followed by smaller red patches shaped like a Christmas tree.
A study published November in Oman Medical Journal confirms cases of the skin conditions pityriasis rosea (PR) and pityriasis rosea-like eruption (PR-LE) in patients who received Pfizer-BioNTech’s mRNA vaccine and Oxford-AstraZeneca’s DNA jab for COVID-19.
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood
Pityriasis rosea is a skin condition with a big round or oval "herald patch" followed by smaller red patches shaped like a Christmas tree.
Pityriasis rosea-like eruption refers to a skin rash that mimics the characteristics of pityriasis rosea.
The study looked at one case in which a 19-year-old young man “with no previously known health issues presented with a two-month history of pruritic, painless erythematous skin eruption over the trunk and the proximal extremities.”
The man “said that the symptoms began one week after receiving the first dose of Pfizer-BioNTech, an mRNA COVID-19 vaccine.”
He was initially treated with oral antihistamine and topical steroids, “with no significant benefit.”
The study detailed his symptoms:
Skin examination showed erythematous, non-tender scaly papules and plaques that followed the skin tension lines over the lateral sides of the chest and coalesced in a reticular pattern over the lower back and abdomen. There was no petechial rash or wheals, and clinical examination revealed no evidence of a herald patch.
A skin biopsy microscopy revealed spongiotic dermatitis—an inflammatory skin condition characterized by fluid buildup, leading to dry, red, itchy, and cracked skin—with mild parakeratosis, another unusual skin condition.
The man was given a course of the oral antibiotic azithromycin at 500 mg once daily for three days and topical corticosteroids were prescribed.
The eruption cleared after two weeks.
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood
In a second case, a woman in her thirties “with no previously known health issues” presented with a one-week history of an erythematous pruritic skin eruption on her trunk, arms, and armpit.
The study authors explain that the eruptions “had begun two weeks after receiving the first dose of Pfizer-BioNTech mRNA COVID-19 vaccine.”
Azithromycin and topical corticosteroids were prescribed, and the eruption cleared in three weeks.
Significantly, the woman later took a second dose of the same vaccine, “and a milder eruption occurred.”
A third case involved a man in his seventies with a history of hand dermatitis, which had been “well-controlled by topical therapy.”
The man presented with a two-month history of new-onset pruritic eruption that started on the trunk and then spread to the extremities.
“Three weeks before the eruption, he had received his second dose of Oxford-AstraZeneca COVID-19 vaccine,” according to the study.
The man was given azithromycin and an antihistamine.
The study authors cited a registry-based study conducted in the U.S. between December 2020 and February 2021.
That study identified 414 skin reactions to Pfizer’s (17%) and Moderna’s (83%) mRNA-based COVID-19 vaccines.
The authors also cited a four-month-long nationwide study in Spain that identified 405 instances of similar reactions after various types of COVID-19 vaccinations: “BNT162b2 (Pfizer-BioNTech; mRNA): 40.2%; mRNA-1273 (Moderna; mRNA): 36.3%, and AZD1222 (AstraZeneca; viral vector): 23.5%.”
In their conclusion, the authors confirm the three cases of PR and PR-LE “were possible cutaneous reactions to COVID-19 vaccination.”
Pfizer Inc.’s safety data indicate the company and the U.S. Food & Drug Administration (FDA) were aware that “pityriasis” was linked to the jab before making it available to the public.

Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood
The study authors’ affiliations include:
Dermatology Residency Training Program, Oman Medical Specialty Board, Muscat, Oman
Dermatology Department, Sultan Qaboos University Hospital, Muscat, Oman
Pathology Department, Sultan Qaboos University Hospital, Muscat, Oman