Cancer-Linked Fat Loss Drug Ozempic to Be Given to U.K. 6-Year-Olds: Read FDA Package Insert
Semaglutide linked to tumors, death, strokes, fertility complications including miscarriages, and heart, pancreas, kidney, and gallbladder diseases.
Hundreds of U.K. children as young as six will be receiving the powerful injectable weight loss and type 2 diabetes treatment drug Ozempic (Semaglutide), which is tied to cancer, death, strokes, fertility complications including miscarriages, and heart, pancreas, kidney, and gallbladder diseases.
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood
More than 200 children will be recruited as part of a global study involving four National Health Service (NHS) hospitals in Birmingham, Bristol, Leeds, and Liverpool.
“One arm of the trial will consist of children aged between six and 12,” while, the second “will involve kids up to 17,” the U.K.’s Daily Mail reports.
Children given semaglutide will “need to be obese or have at least one weight-related health issue, such as type 2 diabetes or high blood pressure.”
Semaglutide has been available for U.K. adults since September 2023, while the U.S. Food and Drug Administration (FDA) approved the drug for American adults with diabetes in 2017 and for weight loss in 2021.
The two-and-a-half-year U.K. trial will be sponsored by Danish pharmaceutical company Novo Nordisk, which is owned primarily by parent company Novo Nordisk Foundation and BlackRock Inc.
Both the Novo Nordisk Foundation and BlackRock are official partners of the “Great Reset”-advancing globalist consortium the World Economic Forum (WEF).
WEF partners are “are the driving force behind the Forum’s programmes,” according to the organization’s website, which include promoting China, the climate change narrative, and Diversity, Equity, and Inclusion (DEI) efforts.


Semaglutide will be given to children despite the drug’s link to many diseases.
Novo Nordisk received FDA approval in 2010 for a similar injectable diabetes treatment drug it also made called Liraglutide.
Liraglutide was subsequently approved for the treatment of obesity in 2014.
Both Semaglutide and Liraglutide work as an analog of human GLP-1 and act as a GLP-1 receptor agonist.
GLP-1 stands for glucagon-like peptide 1, where the “-glutide” part in both drug names comes from.
The molecular formula for Semaglutide is C187-H291-N45-O59.
The molecular formula for Liraglutide is similar: C172-H265-N43-O51.
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood
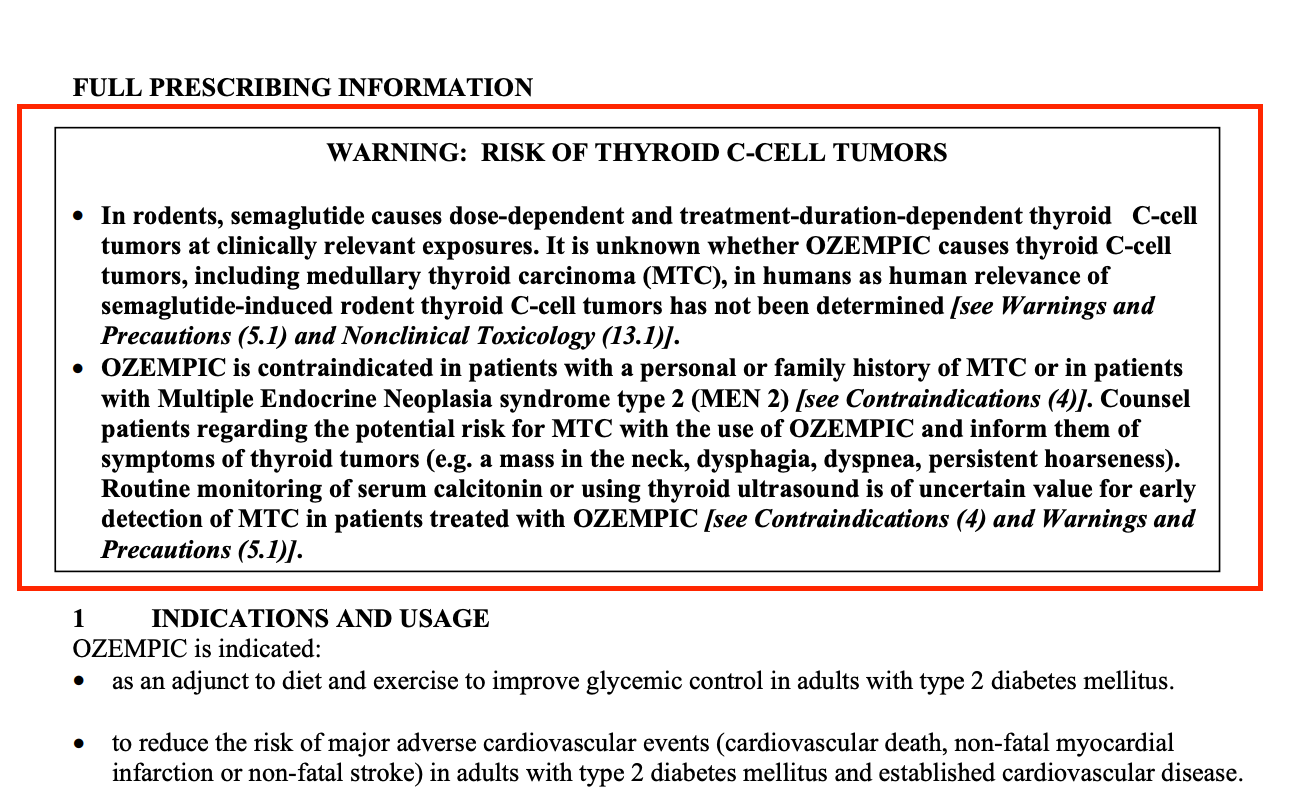
Cancer
The older Novo Nordisk drug Liraglutide has been linked to medullary thyroid carcinoma (MTC), a rare type of thyroid cancer in humans.
Semaglutide has directly “caused” thyroid C-cell tumors (adenomas and carcinomas) in mice and rats.
The Semaglutide package insert also confirms cases of MTC in patients treated with sister drug Liraglutide “have been reported in the postmarketing period.”
Despite this, the insert does not indicate that Novo Nordisk has conducted any clinical or nonclinical studies that could confirm or exclude a causal relationship between Semaglutide and cancer.
The Semaglutide insert simply repeats a cancer warning throughout the document.
On page 1:

Page 3:
Pages 4 and 5:
Page 27:
Pancreatitis
Pancreatitis, inflammation of the pancreas, has been reported in clinical trials for Semaglutide.
Pancreatitis was confirmed in nineteen Semaglutide-treated patients, according to the package insert.
Fertility
In female rats, all Semaglutide dose levels led to an increase in estrus cycle length, together with a small reduction in number of corpora lutea.
The corpus luteum is a temporary endocrine structure in female ovaries that produces progesterone, necessary for the establishment and maintenance of pregnancy.
Pregnant rats, rabbits, and monkeys administered Semaglutide suffered miscarriages, structural abnormalities, and alterations to growth.
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood
Breastmilk, Breastfed Infants
According to the Semaglutide insert, there have been no studies testing the presence of semaglutide in human milk, nor on the drug’s effects on breastfed infants, nor on milk production.
However, Semaglutide was present in the milk of lactating rats.
Other Diseases Linked to Semaglutide
Other diseases noted in the Semaglutide FDA insert are:
Diabetic Retinopathy Complications
Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin
Acute Kidney Injury
Hypersensitivity
Acute Gallbladder Disease
One multi-center, multi-national, placebo-controlled, double-blind cardiovascular outcomes trial confirmed Semaglutide’s link to death, myocardial infarction (death of heart muscle tissue), and stroke.
You can download the Semaglutide (OZEMPIC) FDA package insert below:
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood





Your link to wegovy says 2021, then 2017 for semaglutide, with wider use 2019, the first SINCE a 2014 rx. Which rx was that? Also, though IMHO all injectables are suspect at this point, any injectables which came out after vid jabs and didn’t separate the two groups, which no one is doing, may be vid jab reactions that continue to be buried and blamed on other things. From here on out ‘clinical trials’ are confounded by them so results are even further untrustworthy.
Another toxic "medication".