Cancer Eradicated in Every Patient Using Monoclonal Antibody Treatment: Resurfaced 'New England Journal of Medicine' Publication
Successful treatment meant patients avoided chemoradiotherapy and surgery.
A June 2022 publication in The New England Journal of Medicine (NEJM) has resurfaced, describing a prospective phase 2 study in which every rectal cancer patient who was given monoclonal antibody treatment achieved complete disease remission.
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood
The study reappears as the U.S. Food and Drug Administration (FDA) recently granted an Emergency Use Authorization (EUA) for a monoclonal antibody called Pemgarda (pemivibart) to prevent COVID-19 for moderately-to-severely immunocompromised individuals 12 years of age and older.
In the resurfaced study, 12 cancer patients were given dostarlimab, brand name Jemperli, a single-agent anti–PD-1 monoclonal antibody.
Monoclonal antibodies are laboratory-produced proteins that mimic the immune system’s ability to bind to specific targets, such as cancer cells or infectious agents.
Following the treatment, cancer was completely removed in every patient.
“All 12 patients (100%; 95% confidence interval, 74 to 100) had a clinical complete response, with no evidence of tumor on magnetic resonance imaging, 18F-fluorodeoxyglucose–positron-emission tomography, endoscopic evaluation, digital rectal examination, or biopsy,” the study reads.
Dostarlimab was administered every 3 weeks for 6 months.
Although the antibody treatment was to be followed by standard chemoradiotherapy and surgery, no patient was required to do so because they all had a clinical complete response to the treatment.
“At the time of this report, no patients had received chemoradiotherapy or undergone surgery, and no cases of progression or recurrence had been reported during follow-up (range, 6 to 25 months),” the publication authors write.
Significantly, no patient in the clinical trial observed any severe, life-threatening, or fatal adverse events from the treatment.
“No adverse events of grade 3 or higher have been reported,” the NEJM article confirms.
You can download the full study here:
At the time, The Epoch Times’ Roman Balmakov ran a segment on the study on his ‘Facts Matter’ YouTube show:
Video source: YouTube/@FactsMatterRoman
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood




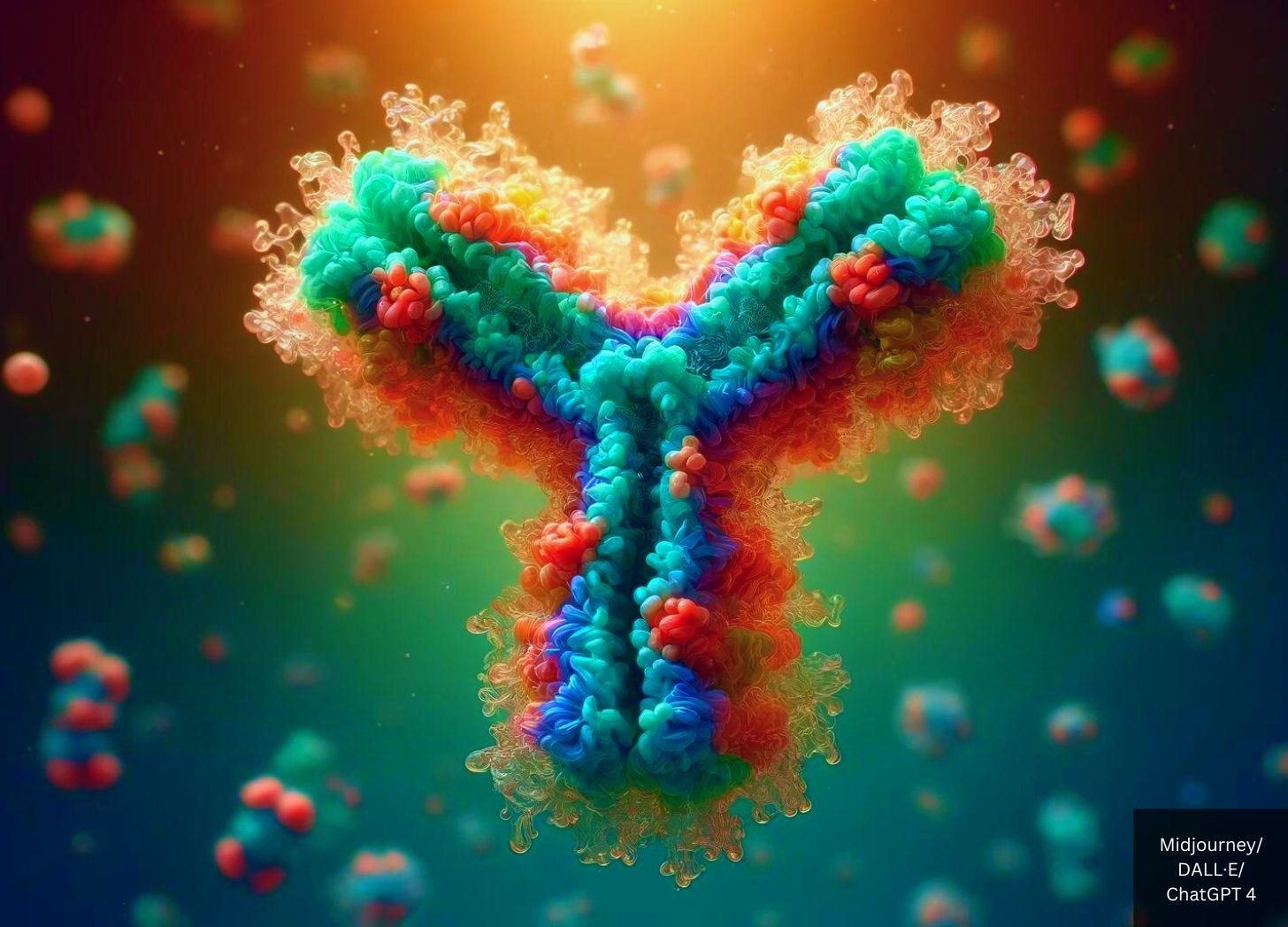
Dr. Carrie Madej said monoclonal antibodies were used against cancer successfully but the patient died later. When they opened up the body the organs were melted together. She said this to say that monoclonal antibodies from humanized mice cells might be a valid treatment for covid because one or two treatments are needed. She also meant this as a warning to not trust the treatment. You can see a video of her at PastorButch.com where she talks about Covid.
That sounds like great news! FACT: Chemo and radiation treatments are more often then not - a death sentence!